New species of Scalibregmatidae (Annelida, Polychaeta) from the East Antarctic Peninsula including a description of the ecology and post-larval development of species of Scalibregma and Oligobregma PDF
Preview New species of Scalibregmatidae (Annelida, Polychaeta) from the East Antarctic Peninsula including a description of the ecology and post-larval development of species of Scalibregma and Oligobregma
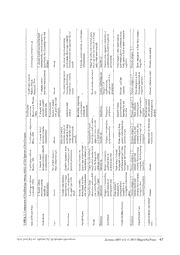
Zootaxa 4033 (1): 057–093 ISSN 1175-5326 (print edition) Article ZOOTAXA www.mapress.com/zootaxa/ Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4033.1.3 http://zoobank.org/urn:lsid:zoobank.org:pub:9C0A63B6-5532-484D-BBD7-EDD5250D4ABA New species of Scalibregmatidae (Annelida, Polychaeta) from the East Antarctic Peninsula including a description of the ecology and post-larval development of species of Scalibregma and Oligobregma JAMES A. BLAKE1,2 1Aquatic Research & Consulting, 24 Hitty Tom Road, Duxbury, MA 02332 USA. 2University of Massachusetts, Boston, 100 Morrissey Blvd., Boston, MA 02125 USA. E-mail: [email protected] Abstract A large collection of scalibregmatid polychaetes from the east Antarctic Peninsula in May 2000 has yielded specimens of three new species of Scalibregma, Pseudoscalibregma, and Oligobregma. The new species of Scalibregma is represented by more than 400 specimens that include post-larval and juvenile forms which, for the first time, provide data on the se- quence of development of key characters of a scalibregmatid. These data demonstrate that taxonomic characters including the form of the prostomium and presence of branchiae develop late in ontogeny and that small specimens cannot be reli- ably referred to a species or genus without a growth sequence. Juvenile morphology is also presented for the new species of Oligobregma. The new species of Scalibregma is compared with five northern hemisphere species and differs in details of the peristomium, upper and lower lips of the mouth, dorsal and ventral cirri, and nature of the short spinous setae of setiger 1. The new species of Pseudoscalibregma is unique in the nature of asymmetrical ventral cirri of posterior setigers. The new species of Oligobregma has large acicular spines in both noto- and neuropodia and these are present in juveniles. However, the final adult configuration of the prostomium is not evident until late in development. The taxonomic signif- icance of the timing of development of post-larval and juvenile morphology elucidated in this study is discussed in relation to the validity of certain taxa and the current system of genera used in the family. Key words: Scalibregmatidae, Pseudoscalibregma, new species, Antarctica, Larsen Ice Shelf, Weddell Sea; reproduction, juvenile morphology Introduction Scalibregmatids are burrowing infaunal deposit feeding worms that are widely distributed but not commonly collected. They range from the intertidal to the deep-sea, with most species occurring deeper than 1000 m. Superficially, the bodies of most scalibregmatids have a rugged appearance because the cuticle is areolated with up to six annulated rows per segment. Their bodies are either elongate (arenicoliform) or maggot-shaped, and often inflated anteriorly. They typically have a bifid or T-shaped prostomium with frontal horns, which for Polyphysia crassa (Örsted, 1844) has been found to assist burrowing through the sediment (Elder 1973). Scalibregmatids have biramous parapodia that bear simple setae, including capillaries, lyrate setae, and acicular spines, some of which are large and conspicuous. Because scalibregmatids largely occur in deep water, little is known about their biology. Scalibregmatids from Antarctic seas are generally well documented (Hartman 1967; Blake 1981; Schüller & Hilbig 2007; Schüller 2008). Collectively, these authors have reported 13 species from Antarctic and subantarctic locations distributed in the genera Asclerocheilus (1), Oligobregma (6), Pseudoscalibregma (3), Scalibregma (1), Sclerocheilus (1), and Axiokebuita (as Kebuita) (1). Of these 13 species, 12 are endemic to the Southern Ocean; the one species of Scalibregma has been referred by these and other authors to S. inflatum Rathke, 1843, the type- species. A large, well-preserved collection of scalibregmatids was collected as part of a geological and biological survey along the eastern side of the Antarctic Peninsula in May 2000. Included among the more than 450 specimens is a full size range representing post-larval forms, juveniles, and fully developed and mature adults of a Accepted by J. Williams: 28 Sept. 2015; published: 21 Oct. 2015 57 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 new species of Scalibregma. Study of the post-larval and juvenile specimens provided information of the developmental sequence of morphology in scalibregmatids for the first time. The new species of Scalibregma is compared with five previously described species, all from the northern hemisphere. In addition, three additional species of Scalibregmatidae are present in the collections, including one new species each of Pseudoscalibregma and Oligobregma and P. bransfieldium (Hartman, 1967) a previously known species. Material and methods The scalibregmatids used for this study were collected during a research cruise to the vicinity of the Larsen-A Ice Shelf (LIS-A) in May 2000 on the RVIB Nathaniel B. Palmer (Table 1). The objective of the survey was to describe the geology of the seafloor and to collect supporting benthic biology. A general account of the cruise including some geological results is found in Domack et al. (2001). Benthic biology samples were mostly obtained with a 0.1 m² Smith McIntyre grab. Each sample was physically divided in half by a Plexiglas divider. One half was used for geological assessment; the other half was for benthic infauna analysis resulting in a sample having a surface area of 0.05 m². Each sample was elutriated and sieved through a 300 µm mesh sieve and preserved in 10% formalin. After the cruise, the samples were shipped from the U.S. Antarctic Program base in Punta Arenas, Chile, to Port Hueneme, California, and from there to J.A. Blake in Woods Hole, MA, where the samples transferred to 70% ethanol. The samples were then sorted to major taxonomic category by a local laboratory, and subsequently distributed to the taxonomic team. A few additional samples were obtained with a megacore having core tubes with a diameter of 10 cm; some of these samples were processed on board the vessel in order to extract living polychaetes that were kept alive in culture dishes in the laboratory, observed, and photographed; other core tubes were preserved in 10% formalin for subsequent analysis. A few specimens of Scalibregmatidae were observed from these cores. For the taxonomic analysis of scalibregmatids, all specimens were examined with light microscopy using a high-quality Wild M-5 stereomicroscope and a Zeiss RA research compound microscope equipped with phase contrast and Nomarski differential interference optics. Photomicrographs were taken with a Nikon D80 camera mounted on both the stereo- and compound microscopes. Some specimens were initially stained with a solution of Shirlastain A in water to highlight difficult-to-see surficial morphology. For this procedure, specimens were left in the stain until fine details of the morphology became evident by viewing with a stereomicroscope. Line drawings were developed with pencil using a drawing tube or Camera Lucida on the Zeiss RA and later transferred to drawing paper and inked. Additional specimens were prepared for scanning electron microscopy (SEM) at Hofstra University by dehydrating in an ascending ethanol series of 70‒95% ETOH for 10 min each, followed by three changes of 100% ETOH for 15 min each. Specimens were critically point dried with a Samdri 795 Critical Point Dryer, mounted on aluminum stubs, coated with gold using an EMS-550 Sputter coater, and viewed with a Hitachi S-2460N SEM or FEI Quanta 250 SEM. Specimens including type material are lodged at the Los Angeles County Museum of Natural History (LACM- AHF Poly); Museum of Comparative Zoology, Harvard (MCZ), and the U.S. National Museum of Natural History (USNM). A specimen from East Antarctica of the new Scalibregma species was from the Australian Antarctic program and is deposited in the Australian Museum (AM). Some non-type specimens are being retained by the author for further study and comparison with related species (JAB). Terminology. Morphological terms used in this paper more or less follow those traditionally used in scalibregmatid descriptions. However, several morphological criteria differ from traditional usage and or have rarely been used in scalibregmatid descriptions. In addition, juveniles described herein differ from adults in several aspects. In order to avoid confusion raised by reviewers of this paper, the following glossary of terms is presented that emphasizes some of the main characters of taxonomic importance used in this article. Acicular spines. In scalibregmatids these large spines are limited to a few anterior notopodia and sometimes neuropodia; spines are curved, usually sigmoid in shape, often covered with fibrils, and terminate in a blunt or pointed tip. These differ from the “spinous setae” described below. Anal cirri. There are typically five or more elongate, fragile cirri located terminally on the pygidium; often lost during preservation. 58 · Zootaxa 4033 (1) © 2015 Magnolia Press BLAKE a. ul s n ni e st Antarctic P ampling Gear M M, MC M M, Dredge M M, MC M, MC M M M M M M M M M M M M M M M M M M M M M M M M M a S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S S E el, n n a h v C m) e Gusta Depth ( 768 504 385 668 978 733 839 305 332 350 317 323 419 776 713 719 665 879 899 912 868 901 628 564 684 794 690 843 887 587 865 651 c n Pri d n a helf A de-W 678´ 911´ 694´ 033´ 720´ 720´ 771´ 378´ 392´ 281´ 459´ 836´ 438´ 786´ 745´ 662´ 498´ 846´ 345´ 450´ 557´ 566´ 459´ 226´ 976´ 942´ 637´ 985´ 025´ 857´ 140´ 505´ n Ice S Longitu 058°34. 058°37. 059°30. 060°32. 060°10. 060°10. 060°04. 060°07. 060°13. 060°19. 060°24. 060°28. 060°33. 060°36. 059°55. 060°07. 059°59. 060°20. 060°22. 060°19. 060°21. 060°21. 059°38. 059°13. 058°36. 058°30. 058°26. 058°26. 058°40. 058°41. 058°34. 058°28. e s ar L 0, 0 May 20 ude-S 7.625´ 8.387´ 3.533´ 9.209´ 6.520´ 5.518´ 3.523´ 7.314´ 7.368´ 6.669´ 5.101´ 3.517´ 1.818´ 8.833´ 3.897´ 9.793´ 9.381´ 2.778´ 4.984´ 5.827´ 6.632´ 7.144´ 3.314´ 9.564´ 2.934´ 2.018´ 1.361´ 6.875´ 6.877´ 1.959´ 0.995´ 0.471´ -3), Latit 64°1 64°1 64°5 64°4 64°4 64°4 64°4 64°5 64°5 64°5 64°5 64°5 64°5 64°4 64°4 64°3 64°3 64°4 64°4 64°4 64°4 64°4 64°4 64°3 64°2 64°2 64°2 64°1 64°1 64°1 64°1 64°1 0 0 P B N ( e y y y y y y y y y y y y y y y y y y y y y y y y y y y y y y y y s a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a a ui e M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M M r Cr Dat 14- 15- 15- 16- 17- 17- 18- 18- 18- 18- 19- 19- 19- 19- 19- 19- 20- 20- 20- 20- 20- 21- 22- 23- 23- 23- 24- 24- 24- 24- 24- 25- e m al P B. el e. nior ac ha hic Sampling Stations—RVIB Nat2); MC, meg-McIntyre Grab (0.1 mLocation Prince Gustav Channel Near Cape Longing Off Lindenberg Island Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Larsen B-transect) Larsen-A (Larsen B-transect) Larsen-A (Larsen B-transect) Larsen-A (Larsen B-transect) Larsen-A (Larsen B-transect) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Larsen-A (Greenpeace Trough) Prince Gustav Channel Prince Gustav Channel Prince Gustav Channel Prince Gustav Channel Prince Gustav Channel Prince Gustav Channel Prince Gustav Channel Prince Gustav Channel Bentmith TABLE 1. Gear: SM, SStation NBP-01 NBP-02 NBP-03 NBP-04 NBP-05 NBP-06 NBP-07 NBP-09 NBP-10 NBP-11 NBP-12 NBP-13 NBP-14 NBP-15 NBP-16 NBP-17 NBP-18 NBP-19 NBP-20 NBP-21 NBP-22 NBP-23 NBP-25 NBP-26 NBP-27 NBP-28 NBP-29 NBP-30 NBP-32 NBP-33 NBP-34 NBP-35 NEW SPECIES OF ANTARCTIC SCALIBREGMATIDAE Zootaxa 4033 (1) © 2015 Magnolia Press · 59 Annulated rings. The segments of scalibregmatids bear 1–6 annular rings composed of separate raised or elevated pads or blocks. The number of rings and the size and organization of the pads are of taxonomic importance. Histologically, the raised pads comprising these annular rings are entirely different from the surficial papillae of the closely related Travisia species. Arenicoliform. Body-shape where the anterior end is expanded, narrowing posteriorly. Branchiae. In scalibregmatids, branchiae are limited to a few anterior parapodia and organized into clusters of dichotomously or pectinately branched filaments. Dorsal and ventral cirri. Cirri of various shapes and forms occur on some genera and are limited to middle and posterior setigers. Fusiform shape. Body shape where the middle of the body is expanded and the anterior and posterior ends are narrow. Frontal and lateral horns. These are outgrowths of the anterior or subapical margins of the prostomium, often providing the prostomium with a T-shaped appearance. These horns are situated on a wedge-shaped anterior end that supports burrowing not feeding. These are superficially similar in appearance to prostomial or lateral horns of some genera of Spionidae. For scalibregmatid species where these horns are exceptionally long, ciliated and apparently grooved, they have been termed “palps” and have been determined to have a role in feeding rather than burrowing. Lyrate setae. These are forked setae located anterior to capillaries having two thin branches with equal or unequal tynes tapering to filamentous tips and with numerous delicate plate-like bristles on the inner margins. Maggot-shaped. Body shape where the entire body is thick from anterior to posterior with no part being noticeably wider or narrower; also termed sausage-shaped. Pygidial lobes. A ring of lobes surrounding the pygidium; number variable from 3 to many; usually with some lobes bearing anal cirri. Spinous setae. These are short, spines located anterior to capillaries or acicular spines on a few anterior segments of adults where lyrate setae do not occur; these are considered to be homologous to lyrate setae because they occupy the same position as lyrate setae on following segments and some are described with split or forked tips, other are blunt or aristate. In juveniles, these spinous setae occur on a greater number of segments and prior to the appearance of any lyrate setae. When lyrate setae do appear, they replace the spinous setae except for those anterior segments where lyrate setae do not develop. Tubular glands. Unicellular tubular glands are present in dorsal and ventral cirri of some species; similar glands have been reported in clusters on some anterior noto- and neuropodia. Upper and lower lips of the mouth. The upper and lower lips of the mouth are arranged in groups of elevated lobes or pads (forms are species-specific).. The upper lip is entirely derived from the peristomium; the lower lip is also derived from the peristomium, but may have contributions from the first setiger. Ventral groove. A distinct mid-ventral groove containing 1–4 raised pads per segment forming a ridge line is present on species described in this paper. Taxonomic account Family Scalibregmatidae Malmgren 1867 Genus Scalibregma Rathke, 1843 Type Species: Scalibregma inflatum Rathke, 1843. Synonym: Oligobranchus Sars, 1846. Fide Hartman 1959. Diagnosis. Body elongate, arenicoliform. Prostomium T-shaped with lateral horns. Peristomium achaetous, surrounding prostomium dorsally and forming upper and lower lips of mouth ventrally. Parapodia of posterior segments with dorsal and ventral cirri; interramal papilla present; postsetal lamellae absent. Branchiae present in anterior segments. Setae include capillaries, lyrate setae, and sometimes inconspicuous blunt, pointed, or bifurcated spinous setae anterior to capillaries of setigers 1 or 1‒2, representing homologues of lyrate setae; large conspicuous acicular spines absent. Pygidium with long anal cirri. 60 · Zootaxa 4033 (1) © 2015 Magnolia Press BLAKE Remarks. Until recently, Scalibregma inflatum was considered to be cosmopolitan in its distribution. Mackie (1991), however, demonstrated sufficient variability in European populations to define an additional, closely related species, S. celticum Mackie, 1991. Among other observations, Mackie (1991) discovered that short slender spines were present anterior to the capillaries of a few anterior noto- and neuropodia anterior to the setigers where lyrate setae occurred. Prior to this study Scalibregma had been defined as lacking any type of spinous seta. The spinous setae discovered by Mackie were not the large, curved acicular spines that have been reported for species of Asclerocheilus, Oligobregma, Parasclerocheilus Sclerobregma, and Sclerocheilus but were instead inconspicuous companions of the capillaries. For S. inflatum Mackie (1991) found that some of these setae were forked or split on their tips. This observation plus their position in the setal fascicles suggested that they were homologous to the lyrate setae of following segments. Mackie further suggested that the larger recurved spines of other genera were homologous with capillaries. Mackie (1991) redescribed Scalibregma inflatum based on specimens from the type locality in Norway as well as from Sweden, Scotland, Wales, and Ireland. The second species, S. celticum, was from Scotland, Wales, and France and differed in that the small spinous setae of setigers 1‒2 were blunt-tipped instead of bifurcate. Additionally, eyes were present instead of absent and there were differences in the form of the peristomium, size and distribution of the epidermal pads above the notopodia, and in the number and arrangement of the pygidial cirri. S. celticum was subsequently reported from the Mediterranean by Çinar (2005) and Lomiri et al. (2012). Mackie (1991) also re-examined Sclerobregma stenocerum Bertelsen & Weston, 1980, from shelf depths along the southeastern United States and found that the anterior acicular spines reported for the species by Bertelsen & Weston (1980) were of the small, inconspicuous kind found in Scalibregma species instead of the large, curved acicular spines found on other species of the Sclerobregma. S. stenocerum was therefore transferred by Mackie (1991) to Scalibregma. Mackie also examined the holotype of S. branchiatum Hartman, 1965, the type-species of Sclerobregma from deep water in the western North Atlantic and found short spinous setae anterior to the larger acicular spines of setigers 1 and 2. A similar situation exists in Cryptosclerocheilus baffinensis Blake, 1972, described from deep water in Baffin Bay. This species was reported to have slender, blunt-tipped spines in the noto- and neuropodia of setiger 2; these were replaced by furcate setae from setiger 3 (Blake 1972). A re-examination of prepared slides of these spines confirms that these are the same type of seta reported by Mackie (1991) for the three species of Scalibregma examined by him. Following the lead of Mackie (1991), Blake (2000) examined specimens from California previously identified as Scalibregma inflatum and described a new species, S. californicum Blake, 2000. He also suggested that specimens from the U.S. Atlantic coast included at least one new species in addition to S. stenocerum. Most recently, Bakken et al. (2014) described S. hanseni Bakken, Oug & Kongsrud, 2014 from deep water off Norway. These authors also focused on the short, spinous setae anterior to normal capillaries in setigers 1‒2 and found similar spines in Pseudoscalibregma parvum (Hansen, 1879). In my own work, numerous species of Scalibregmatidae have been examined and these short spinous setae, considered homologous to the lyrate setae, have been found in other genera including some with large curved acicular spines. I now believe that most species of Scalibregmatidae having lyrate setae will be found to have the same type of short spinous setae in segments anterior to where the lyrate setae begin. To date, specimens of Scalibregma from Antarctic waters have been identified in several faunal and ecological accounts as S. inflatum (see reference list below). The only original illustrations of Scalibregma from Antarctica appear to be by Knox & Cameron (1998) of the anterior end and a branchiate parapodium from a specimen collected from the Ross Sea in 578 m. Scalibregma australis new species Figures 1‒3 Scalibregma inflatum: Hartman 1967: 134, 1978: 181; Blake 1981: 1146; Siciński 1986: Table II, Fig. 5, 2000: 164, 2004: 82; Hartmann-Schröder 1986: 84; Hartmann-Schröder & Rosenfeldt 1989: 73, 1991: 75; Cantone & Sanfilippo 1992; Knox & Cameron 1998: 75, figs 141‒142; Cantone 1994: 41; Cantone et al. 2000: 554; San Martín et al. 2000: 85, 91; Lovell & Trego 2003: 1813; Montiel et al. 2005: 199; Hilbig et al. 2006: 724; Schüller et al. 2009: 63; Barbosa et al. 2010:1155; Parapar et al. 2011a: 728; Pabis & Sobczyk 2015: 115‒117. Not Rathke 1843. NEW SPECIES OF ANTARCTIC SCALIBREGMATIDAE Zootaxa 4033 (1) © 2015 Magnolia Press · 61 Material examined. East Antarctic Peninsula, RVIB Nathaniel B. Palmer Cruise 2000-03, Collector, J.A. Blake.—Prince Gustav Channel, Sta. NBP-01, 768 m, 62 paratypes (LACM-AHF Poly 7001); Sta. NBP-27, 684 m, 12 paratypes (USNM 1281913); Sta. NBP-28, 794 m, 4 specimens (JAB); Sta. NBP-29, 690 m, 11 paratypes (LACM-AHF Poly 7007); Sta. NBP-30, 843 m, 13 specimens (JAB); Sta. NBP-33, 587 m, 19 specimens (JAB); Stas. NBP-35, NBP-35A, NBP-35B, 651 m, 3, 6, and 14 specimens, respectively, from three grabs (JAB).—Off Cape Longing, Sta. NBP-02, 504 m, 19 paratypes (LACM-AHF Poly 7002).—Off Lindenberg Island, Sta. NBP-03, 385 m, 12 paratypes (USNM 1281909).—Larsen Ice Shelf Area, Greenpeace Trough, Sta. NBP-04, 668 m, holotype and 17 paratypes (LACM-AHF Poly 7003, 7004); Sta. NBP-05, 798 m, 7 specimens (JAB); Sta. NBP-06, 733 m, 7 paratypes (LACM-AHF Poly 7005); Sta. NBP-07A, 839 m, 8 paratypes (USNM 1281910); Sta. NBP-07B, 839 m, 15 paratypes (MCZ 60891); Sta. NBP-16, 713 m, 14 paratypes (MCZ 60892); NBP-17, 719 mm, 7 paratypes (MCZ 60893); Sta. NBP-18, 665 m, 11 specimens (JAB); Sta. NBP-19, 879 m, 2 specimens (JAB); Sta. NBP-20, 899 m, 1 specimen (JAB); NBP-22, 3 specimens (JAB); Sta. NBP-23, 901 m, 7 specimens (JAB).—Larsen Ice Shelf Area, transect along border with Larsen Ice Shelf B, Sta. NBP-10, 332 m, 18 paratypes (USNM 1281911); Sta. NBP-11, 350 m, 3 specimens (JAB); Sta. NBP-12, 317 m, 27 paratypes (LACM-AHF Poly 7006); NBP-13, 323 m, 16 paratypes (USNM 1281912); Sta. NBP-14, 8 specimens (JAB).— Weddell Sea off LIS-A Area, Sta. NBP-25, 628 m, 9 specimens (JAB).—East Antarctica, Wilkes Land, Vincennes Bay, Casey Station, coll. Australian Antarctic Division, O’Brien Bay, SRE-1, Control R2, 12 Nov 1997, 66.295°S; 110.536°E, diver cores, 12–25 m, 1 specimen (AM). Description. A large species, body elongate, arenicoliform, expanded in anterior half to variable degree, usually from about setiger 4‒5 continuing to mid-body, then tapering to narrow abdominal region (Fig. 3C). Body surface covered with numerous annulated rings; most annulations formed of separate elevated pads or blocks (Figs. 1A–E, 2A). Holotype from Sta. NBP-04 ovigerous female, 32 mm long, 4.5 mm wide across expanded anterior region, with 40 setigerous segments; large paratype from Sta. NBP-01, 21 mm long, 4.5 mm wide anteriorly, for 41 setigers; six smaller paratypes from same sample, 7‒10 mm long, 1.1‒2.1 mm wide for 32‒40 setigers. Numerous smaller specimens including post-larval forms as small as 1 mm long or less with as few as 10 setigers common in samples. Color in alcohol light tan with no yellow-orange caste as in other species; some specimens with distinct dark reddish-colored glands forming a row across dorsum of setigers 4‒5 (Fig. 3A); similar isolated pigmented glands consisting of numerous twisted tubules on some anterior neuropodia (Fig. 2B); these glands likely retaining color derived from Rose Bengal stain used in sample processing; similar reddish-colored glands in dorsal and ventral cirri of posterior parapodia (Figs. 2C–D, 3D–G). Prostomium T-shaped, with lateral processes or horns well developed, pointed laterally, sometimes oriented dorsally or anteriorly (Figs. 1A–C, 2A, 3A–C); posterior margin of prostomium visible dorsally (Fig. 1A–B); eyes absent. Nuchal organs not everted on any specimens; ciliated grooves apparent between prostomium and peristomium on some specimens. Peristomium achaetous, not concealing posterior margin of prostomium, consisting of single ring complete dorsally (Figs. 1A–C), split into three rings ventrally forming the upper lip of the mouth and together with annulated rows from setiger 1 forming the lower lip of the mouth (Figs. 2A, 3B). Mouth surrounded by broad lateral lips divided anteriorly into a row of small elongate lobes forming upper lip of mouth and posteriorly by 4–5 large paired lobes or blocks forming lower lip of mouth at level of setiger 1 (Fig. 2A). Juvenile morphology suggests that upper and lower lip morphology entirely derived from peristomium (see below). Proboscis smooth sac when everted. Dorsally, setiger 1 biannulate and setigers 2‒3 triannulate (Figs. 1A–B); ventrally, setigers 1–3 triannulate (Fig. 2A); subsequent anterior setigers of expanded region quadriannulate, narrow posterior segments initially quadriannulate, then becoming pentannulate and quadriannulate in far posterior setigers (Fig. 1D). Each annulation divided into separated elevated pads or square-shaped blocks, providing complex areolated appearance to body surface. Ventral midline from setiger 2 with group of four large epidermal pads per segment in anterior segments (Figs. 2A, 3B), merging into a single pad in middle and posterior segments, forming ridge line within mid-ventral groove; mid-ventral groove and ridge line continuing posteriorly (Fig. 3C). Arborescent or dendritically branched branchiae present on setigers 2‒5 posterior to notosetae (Figs. 1A, C, 2A–B, 3B). Parapodia reduced, inconspicuous anteriorly (Figs. 1C, 2B) becoming larger and more conspicuous in middle and posterior setigers (Figs. 1D, 2C–D); Dorsal and ventral cirri develop from about setigers 17‒20 or mid- body; dorsal cirri initially short, triangular (Figs. 2C, 3D), becoming slightly longer and narrower by about setiger 30 (Figs. 1E, 2D, 3F), continuing to posterior end; ventral cirri narrower than dorsal cirri, oval, tapering to rounded 62 · Zootaxa 4033 (1) © 2015 Magnolia Press BLAKE tip. Each dorsal and ventral cirrus with darkly pigmented glands; each gland formed of tubules appearing to exit dorsally on dorsal cirri and ventrally on ventral cirri (Figs. 3D‒G). Interramal papillae small, inconspicuous in anterior setigers, becoming larger in middle and posterior parapodia (Fig. 2C). All setigers with noto- and neuropodial fascicles of slender capillaries, with those of anterior fascicles more numerous, arranged into 2‒3 rows, with setae of posterior row longest; capillaries of middle and posterior setigers arranged in 1‒2 rows; all capillaries with numerous short bristles along length, representing emerging fibril endings (Figs. 1F–G); setiger 1 with additional anterior row of inconspicuous short, slender, pointed aristate spinous setae (Figs. 1F–G; 2E) with occasional spine having two thin branches (Fig. 2E); spines numbering 8‒12 on large specimens, fewer on specimens of 10 mm or less in length. Setigers 2 and following with lyrate setae in same anterior position as short spines of setiger 1; each lyrate seta with nearly equal tynes tapering to filamentous tips and with numerous flattened, plate-like bristles on inner margins (Fig. 1H–I, 2F), details best seen with SEM. Pygidium of largest specimens with anal opening surrounded by about 12 elongate lobes (Fig. 1D), pygidium inflated in smaller specimens with fewer poorly defined lobes poorly defined (Fig. 2G); pygidium with five long, thin anal cirri, two dorsal, two ventral and one mid-ventral (Fig. 2G); these cirri fragile, missing on most specimens. Ecology. Sediment particle size distributions analyzed from the same grabs as the biology samples from vertical subcores taken to a depth of 12 cm were reported by Gilbert & Domack (2003). A core closest to the Antarctic Peninsula in the LIS-A area (Sta. NBP-05) was dominated by silt and clay, but exhibited higher concentrations of coarse sand and gravel of up to 30% near the surface; adjacent cores taken further offshore (Stas. NBP-06 and -07) had a more uniform particle size distribution of fine silt with depth. Out of 16 subcores analyzed from the LIS-A area, ten showed the maximum amount of gravel to occur in the upper 2.5 cm; of the remaining six subcores, four showed a decrease of coarse particles at the surface and two had subsurface maxima. Of nine subcores collected from the Prince Gustav Channel, seven had a sand-gravel maximum at the surface. Of the 26 subcores collected and analyzed for particle size, 65% had a sand-gravel maximum near the surface overlying fine silt and clay. With respect to diversity, the dominance of silt + clay overlain with coarse sediments provides a variable sedimentary habitat to support an interesting mix of benthic invertebrates (Blake & Maciolek unpublished data). Out of 32 successful grab samples (21 from the LIS-A area; 11 from the Prince Gustav Channel), a total of 270 species of benthic invertebrates were identified, 128 of which were polychaetes. Of the total invertebrate fauna, Scalibregma australis n. sp. was the fifth most abundant species identified, with a total of 419 specimens. Two cirratulid polychaetes, Tharyx homosetosus (Hartmann-Schröder & Rosenfeldt, 1989) (891 specimens) and Chaetozone sp. 1 (828 specimens), a bivalve, Yoldiella cf. vallettei (Lamy, 1906) (704 specimens) and a scaphopod, Siphonodentalium sp. (688 specimens) were the four most abundant species. Elsewhere in Antarctica, high benthic biomass (1.3 g/0.1 m2) and high density (37.3 ± 20.6 ind/0.1 m2) for S. inflatum was reported by Pabis & Sobczyk (2015) from a glacial fjord on King George Island, South Shetland Islands having strong currents, away from glacial influence, and with poorly sorted sandy sediments. Typically, scalibregmatid polychaetes do not occur in high densities in benthic communities. Therefore, the dominance of Scalibregma australis n. sp. in sediments along the east Antarctic Peninsula is unusual and reminiscent of similar high densities of S. inflatum in continental slope sediments (600‒1500 m) off Cape Hatteras, NC (Blake 1993; Blake & Hilbig 1994). Live phytoplankton cells were recovered at 14 cm depth in the sediments from the Cape Hatteras sediments, suggesting rapid subduction (Cahoon et al. 1994) and possibly caching by deposit feeders such as S. inflatum. Blair et al. (1996) documented rapid in situ uptake of 13C-labeled Chlorella sp. by S. inflatum and rapid subduction of labeled material from the same area. Similar efforts to understand organic content of sediments along the east Antarctic Peninsula have not been undertaken. R.E. Ruff (personal communication) also provided evidence of high densities of S. californicum in one sample from the NE Pacific in the San Juan Archipelago from 24 m in silt and clay (376 specimens, 14 June 2012) and another from Bellingham Bay, 26 m in silt and clay (683 specimens, 08 Apr 2014). Most scalibregmatids are believed to be burrowing subsurface deposit feeders (Jumars et al. 2015). Available data on the reproduction and post-larval development of S. australis n. sp. is presented in a separate section (below). Remarks. Scalibregma australis n. sp. from Antarctica is compared with five previously known species, all from the northern hemisphere (Table 2). All of the six known species have a T-shaped prostomium with laterally or anteriorly directed fronta l horns, short spinous setae anterior to capillaries in setigers 1 or 2, lyrate setae from NEW SPECIES OF ANTARCTIC SCALIBREGMATIDAE Zootaxa 4033 (1) © 2015 Magnolia Press · 63 FIGURE 1. Scalibregma australis n. sp. SEMs from Sta. NBP 29: A, anterior end, dorsal view; B, same specimen showing details of prostomium, peristomium, and setiger 1; C, anterior end of another specimen, right lateral view; D, posterior end, right lateral view; E, parapodia from posterior setigers, right lateral view; F, notosetae from setiger 1, with anterior row of short spinous setae (arrows) and three rows of capillaries; G, detail of two short neuropodial spinous from setiger 1 and bases of two capillaries with numerous short bristles or fibril endings; H, four lyrate neurosetae from setiger 2; I, same, detail of a single lyrate seta with plate-like bristles along interior margin of tynes. Abbreviations: br, branchiae; dC, dorsal cirrus, frH, frontal horn, intP; interramal papilla; lL, lower lip of mouth; mo, mouth; neP, neuropodium; noP, notopodium; per, peristomium; pr, prostomium; pyg, pygidium; uL, upper lip of mouth, vC, ventral cirrus; vG, ventral groove. 64 · Zootaxa 4033 (1) © 2015 Magnolia Press BLAKE FIGURE 2. Scalibregma australis n. sp. Paratypes Sta. NBP-01 (LACM AHF Poly 7001): A, anterior end, ventral view; B, setiger 4, right side, anterior view; C, mid-body setiger, left side, anterior view; D, posterior setiger, left side, anterior view; E, short spinous setae from setiger 1; F, lyrate seta from middle body segment; G, pygidium, ventral view. Abbreviations: aC, anal cirri; br, branchiae; dC, dorsal cirrus, intP; interramal papilla; lL, lower lip of mouth; per, peristomium; pr, prostomium; pyg, pygidium; uL, upper lip of mouth, vC, ventral cirrus; vG, ventral groove. Numbers 1, 2, & 3 denote three rings of the peristomium. NEW SPECIES OF ANTARCTIC SCALIBREGMATIDAE Zootaxa 4033 (1) © 2015 Magnolia Press · 65 FIGURE 3. Scalibregma australis n. sp. Photomicrographs of Paratypes Sta. NBP-01 (LACM-AHF Poly 7001): A, anterior end, dorsal view; B, anterior end, ventral view; C, entire animal, ventral view; D‒E, dorsal cirri from middle (D) and posterior (E) parapodia showing internal glands; F‒G, ventral cirri from middle (F) and posterior (G) parapodia showing internal glands; H, oocytes, from parts of ovaries; I‒L, oocytes in different stages of maturation; M, sperm packet with sperm nuclei and flagella visible. Abbreviations: dC, dorsal cirrus, vC, ventral cirrus; vG, ventral groove. 66 · Zootaxa 4033 (1) © 2015 Magnolia Press BLAKE
