NASA Technical Reports Server (NTRS) 20030016529: Hydrodynamic Suppression of Soot Formation in Laminar Coflowing Jet Diffusion Flames. Appendix C PDF
Preview NASA Technical Reports Server (NTRS) 20030016529: Hydrodynamic Suppression of Soot Formation in Laminar Coflowing Jet Diffusion Flames. Appendix C
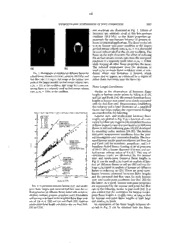
66 Appendix C: Dai, Z. and Faeth, G.M. (2000) "Hydrodynamic Suppression of Soot Formation in Laminar Coflowing Jet Diffusion Flames," Proc. Combust. Inst. 28, 2085-2092. 67 ProceedingsoftheCombustionInstttute, Volume28,2000/pp.2085-2092 HYDRODYNAMIC SUPPRESSION OF SOOT FORMATION IN LAMINAR COFLOWING JET DIFFUSION FLAMES Z.DAI A_'_DG.M. FAETH Department ofAerospace Engineenng University ofMichigan Ann Arbor, MI 48109-2140, USA Effects of flow (hydrodynamic) properties on limiting conditions for soot-free laminar non-premLxed hydrocarbon/air flames (called laminar soot-point conditions) were studied, emphasizing non-buoyant lam- inar coflowmg jet diffusion flames. Effects of air/fuel-stream velocity ratios were of particular interest; therefore, the experiments were carried out atreduced pressures tominimize effects of flow acceleration due to the mtrusion ofbuoyancy. Test conditions included reactant temperatures of 300 K;ambient pres- sures of 3.7--49 8kPa; methane-, acetylene-, ethylene-, propane-, and methane-fueled flames burning in coflowing air with fuel-port diameters of 1.7, 3.2, and 6.4 mm, fuel jet Reynolds numbers of 18-121; air coflow velocities of0--6 m/s; and air/fuel-stream velocity ratios of0.003-70. Measurements included lam- inar soot-point flame lengths, laminar soot-point fuel flow rates, and laminar liftoff conditions. The mea- surements show that laminar soot-point flame lengths and fuel flow rates can be increased, broadening the range offuel flow rates where the flames remain soot free, byincreasing air/fuel-stream velocity ratios. The mechanism of this effect involves the magnitude and direction of flowvelocities relatave tothe flame sheet where mcreased air/fuel-stream velocity ratios cause progresswe reduction of flame residence times in the fuel-rich soot-formation region. The range of soot-free conditions islimited byboth liftoff, particu- larly at lowpressures, and the intrusion of effects ofbuoyancy oneffective air/fuel-stream velocity ratios, particularly at high pressures. Effective correlations of laminar soot- and smoke-point flame lengths were also found in terms of a corrected fuel flow rate parameter, based on simplified analysis of laminar jet diffusion flame structure. The results show that laminar smoke-point flame lengths m coflowing air envi- romnents are roughly twice as long as soot-free (blue) flames under comparable conditions due to the presence of luminous soot particles under fuel-lean conditions when smoke-point conditions are ap- proached. This isvery similar toearher findings concerning differences between laminar smoke- and soot- point flame lengths instill environments. Introduction flame configuration is that it has been widely used to study the soot-formation properties of diffusion Motivated by technological and public health fames (see Refs. [4-8]). problems, several methods have been developed to While fast mixing reduces soot formation within control the soot content and emissions of hydrocar- diffusion flames, past studies of both laminar op- bon-fueled flames. Among these, soot-control meth- posed and coflowing jet diffusion flames show that ods based on fast mixing for non-premixed (diffu- the way that mixing iscarried out is important as well sion) flames are of interest because the), avoid the [9-17]. In fact, existing evidence from both laminar operational problems of additives and premixed and turbulent jet diffusion flames, and from empir- combustion [1-3]. The objective of fast mixing is to ical industrial practice, suggests that soot reductions minimize residence times of fuel and fuel-decom- can be achieved most effectively by ensuring that velocities normal to the flame sheet are directed position products at fuel-rich conditions so that few from the fuel-rich toward the fuel-lean side. This soot particles develop and they can be readily con- configuration, called "soot-formation-oxidation stoned in the soot-o_ddation regions of the flame. flame conditions" by Kang et al. [13], tends to reduce The present investigation seeks improved under- the residence times of sootprecursors and soot at standing of fast mixing concepts based on experi- fuel-rich soot-formation conditions by drawing these mental observations of laminar eoflowing jet diffu- materials directly through the flame sheet toward sion flames. Laminar diffusion flames were studied fuel-lean oxidation conditions. In contrast, when ve- because they provide relatively tractable models of locities normal to the flame sheet are directed from mixing and reaction within more practical but rela- tim fuel-lean toward the fuel-rich side, called "soot- tively intractable turbulent diffusion flames. Another formation flame conditions" by Kang et al. [13], res- advantage of the laminar coflowing jet diffusion idence times of soot precursors andsoot at fuel-rich 2085 68 2086 LAMINARDIFFUSION FLAMES--Coflow JetFlames soot-formation conditions are enhanced, making ox- processes (dominated byconvective transport alone) idation of these materials more problematic when can be controlled to eliminate soot emissions. As- oxidation conditions are finally reached. sociated flame properties such as luminous flame Studies of effects ofvelocities normal to the flame lengths and flame liftoff conditions were also ob- sheet on soot formation have been carried out in served. Finally, present results define conditions laminar opposed and coflowing jet diffusion flames where detailed numerical simulations offlame struc- [9-17]. During most of these studies [9-15], veloc- ture can be evaluated without the complications as- ities normal to the flame sheet were varied byvary- sociated with soot chemistry [18-20]. ing the compositions of the oxidant- and fuel-carry- ing streams. For example, diluting the fuel stream with an inert gas (e.g., nitrogen) while enriching the Experimental Methods oxidant stream byremoving existing diluent (e.g.,re- Measurements were carried out at subatmos- moving nitrogen from air) promotes increased ve- locities normal tothe flame sheet directed from the pheric pressures to control the effects of buoyancy fuel-rich toward the fuel-lean side and yields re- [21]. The test burner was a vertical coaxial tube ar- duced soot concentrations in the flame [9--14]. As rangement with the fuel flowing from an inner port pointed out bySunderland et al.[12],however, these with inside diameters of 1.7, 3.2, and 6.4 mm and composition changes alone are sufficient to retard the air flowing from an outer port with an inside soot formation and enhance soot oxidation, which diameter of60 mm. The airpassage used beads and tends to reduce soot concentrations, obscuring the screens to provide a uniform velocity distribution at effect ofhydrodynamics on sootcontrol. In addition, the burner exit; the fuel passage provided fully de- the practical utility of varying reactant-stream com- veloped laminar flow at its exit. The exit of the fuel positions to control soot formation in diffusion port was 10 mm above the exit of the air port to fames isrelatively limited. provide an undisturbed region forflame attachment. The present investigation sought a direct evalua- The air-port diameter was sufficiently large so that tion of effects ofvelocities normal tothe fame sheet the mixing layer between the air coflow and the am- bient air in the vacuum chamber did not disturb the on soot formation in diffusion flames byconsidering pure air and fuel reactant streams for laminar co- flame. The burner was operated within awindowed flowing jet diffusion flames. Intliisconfiguration, en- vacuum chamber with an inside diameter and length hanced (retarded) airstream velocities provide en- of 300 and 1200mm, respectively. trainment velocities normal to the flame sheet Acetylene-, ethylene-, propane-, and methane-fu- directed from the fuel-rich (fuel-lean) to the fuel- eled laminar jet diffusion flames in coflowing air lean (fuel-rich) sides of the flame, which should re- were considered with gas purities in excess of 99%, duce (increase) both soot concentrations within the except foracetylene, which had apurity of only98% flame and the tendency toemit soot from the flame. due to contamination bythe acetone that ispresent This behavior has been observed, with enhanced air- in commercial acetylene cylinders for safety pur- stream velocities yielding significant increases of poses. Past work has shown, however, that effects of laminar smoke-point flame lengths--particularly for acetone contamination of acetylene on luminous low-pressure flames, in which disturbances of the flame shapes and laminar smoke-point flame lengths velocity field due to the intrusion of effects of buoy- are small compared with experimental uncertainties ancy become relatively small [16]. Recent numerical [16]. In addition to the variations of burner-port di- simulations from Kaplan and Kailasanath [17] ex- ameters and fuels mentioned earlier, test conditions hibit similar tendencies for soot concentrations included reactant temperatures of roughly 100 K; within laminar coflowing jet diffusion flames to de- ambient pressures of 3.7--49.8 kPa; fuel jet exitRey- crease for locally enhanced airstream velocities. Fi_ nolds numbers, Re, of 18-121; air coflow velocities nally, air atomization, which iswidely used for soot of 0--6 m/s; and air/fuel-stream velocity ratios of 0.003-70. Transition to turbulent flames was never control in aircraft gas turbine combustors, corre L sponds to an enhanced airstream velocity flame con- observed during the present experiments, whereas characteristic flame residence times were small so figuration, which may explain dais soot-control mechanism. that effects of radiative heat losses from the flames Prompted by these observations, the present in- were negligible [8,22]. vestigation considered effects ofenhanced airstream velocities on laminar soot-point properties--that is, the condition where soot isfirst observed in laminar Results and Discussion diffusion flames. The main issue was to learn whether gas-phase processes (dominated by both Flame Appearance diffusive and convective transport) could be con- trolled toyield soot-free flames bymanipulating air/ Photographs of typical soot-free (blue) and soot- fuel velocity ratios in the same way that gas/solid containing ethylene/air flames at identical fuel-port 69 HYDRODYNAMIC SUPPRESSION OF SOOT FORMATION 2087 exit conditions are illustrated in Fig. 1. Effects of buoyancy are relatively small at this low-pressure condition (10.2 kPa), so that flame properties ap- proximate the non-buoyant behavior of greatest in- terest for practical applications. The flame on the left is at its laminar soot-point condition at the largest air/fuel-stream velocity ratio, u,/uf = 0.2, that could be used without liftoff at this jet exit condition. The flame on the right illustrates the effect of reducing the air/fuel-stream velocity ratio from the soot-point condition to a relatively small value, uJuf = 0.004, while keeping all other flame properties the same. The reduced entrainment from the airstream at (a) (b) small u,/uf increases flame residence times at con- FIG. 1.Photographs of ethylene/air diffusion flames for ditions where soot for]nation is favored, which afixedburner diameter (3.2ram), pressure (10.2 kPa), and causes soot to appear, as evidenced by a region of fuel flow rate (1.3 mg/s): left image at the laminar soot yellow fame luminosity near the flame tip. point atthe largest possible mr/fuel-stream velocity ratio, uJuf = 0.2, atthis condition; right image for asoot-con- Flame Length Correlations taining flame at a relatwely small air/fuel-stream velocity ratio, uJuf = 0.004, atthis condition. Similar to the observations of luminous flame lengths at laminar smoke points by Schug et al. [5] and Lin and Faeth [14], the present luminous flame lengths at laminar soot points were closely associated with the fuel flow rate. Measurements establishing this behavior and a brief discussion of a simplified theory that helps explain the experimental findings are considered in the following. 100 ! , I I i I Laminar soot- and smoke-point luminous flame SYM FUEL -4 lengths are plotted in Fig. 2 as a function of a cor- • C H SMOKE POINT rected fuel flow rate suggested bysimplified theories 6O •" CC_HH•I FINLASUTEILLLEGNAGSTHANO Frf =0 J of flame shapes for non-buoyant laminar jet diffusion flames in still and coflowing gases [22,23] developed 4 • • by extending earlier analyses [24--26]. The laminar soot-point measurement conditions from the pres- _0 • • • ent investigation were summarized earlier. The mea- sured laminar smoke-point correlations are from Lin _ H and Faeth [14] for acetylene-, propylene-, and 1-3- E 0. • INCOFLOW butadiene-fueled flames burning in air at pressures 40 • (C I);H 1999) of 19--51 kPa, aburner diameter of 6 mm, and air/ fuel-stream velocity rataos of 0.4-6.7. Two sets of _o correlations (each) are illustrated for the laminar _O0"l' POINT soot- and smoke-point luminous flame lengths in 20 • INSTILL GAS Fig. 2: one for small uJuf based on analysis of lam- inar jet diffusion flames m still air [22] and one for • AND F 5) large u_/uf based on analysis of laminar jet diffusion FLAME LENGTH 2 INCOFLOW flames in coflowing air [23]. There are good corre- (ualuf_1 lations between measured luminous flame lengths 0L,- m" , I • I . AND F;'i>1) . ! . and the corrected fuel flow rates for both laminar 0 400 800 1200 ';600 20QO soot- and smoke-point conditions (see Re['. [23] for _d/Za _a) Imm) the latter). As aresult, laminar soot-point properties FIG. 2. Correlations between laminar soot- andsmoke- are represented by the laminar soot-point fuel flow point flame lengths and corrected fuel flow rates for co- rate in the following, similar to past work [14]. It is flowing lmaainarjet diffusion flames fueled with acetylene, also evident that the correlation for laminar smoke- ethylene, methane, propane, propylene, and 1-3-butadiene point flame lengths is roughly twice as long as that andburmng mairbased onthe simplified flame shape anal- for laminar soot-point flame lengths at both large ysis of IAn et al. [22] and Lin and Faeth [23]. Laminar and small uJut limits. smoke-point flame length correlations also are from Refs. An explanataon of the flame length behavior ob- [22] and [23]. served in Fig. 2 can be obtained from the fame 7O 2088 LAMINADRIFFUSIOFLNAMES---CJoefFtlolawmes 2.5 in air environments by using values of the Schmidt number and viscosity for air at the average of the adiabatic flame temperature and the ambient tem- 2.0 perature. Similarly, Cn = 3 for non-buoyant flames in still gases, whereas C, = 2 for non-buoyant flames in coflowing gases [23]. The measurements of Refs. [27] and [28] yield Cr _ 0.5 for soot-free blue flames 1.5 and Cf _ 1.0 for flames at the laminar smoke point for flames in still air [22]. These assignments provide •E- 1.0 the good correlations of the present results in co- flowing air seen in Fig. 2, as well as an explanation of the increased luminous flame lengths caused by reduced air coflow velocities and the presence of 0.5 soot near the flame tip for these conditions seen in Fig. 1. 0.0 0 2 4 6 8 Laminar Soot-Point Properties u=(m/s) Both laminar soot-point and liftoffproperties were measured during the present experiments. The tests FIG. 3 Fuel flow rates at laminar soot-point and liftoff were conducted by varying the pressure range for conditions as a function of air coflow velocities, fuel-port each fuel based on its propensity to soot, so that diameter, and pressure for acetylene/air flames. effects of reasonable variations of air/fuel-stream ve- locity ratios could be measured for flames fueled with each fuel in spite of limitations due to effects 3.0 | I | i ' I . of liftoff and the intrusion of buoyancy. PRESSURE(kPa)" ETHYLENE In the following, effects of air coflow on laminar SYM. d(rnm) 6.8 soot-point and liftoff properties are presented as "s C3 • 3.2 plots of laminar scot-point fuel flow rates as a func- A • 4.8 tion of air coflow velocities because this approach 2.0 yttl OREVE•RSE SH1A.6DED provades a compact presentation of the measure- SYMBOL DENOTES UIIuI =1 ments. Effects of air eoflow velocities on laminar II 10.2 soot-point fuel flow rates were qualitatively similar for the four fuels that were considered. This can be •E- _ 13.5 seen from the plots of fuel mass flow rate at soot- point conditions as afunction of air coflow velocities for the various pressures and fuel-port diameters that are illustrated in Figs. 3-6. To indicate the tran- _e_._._ _ _ A sition between soot-formation and soot-formation- oxidation configurations at the base of the test 0.0 , I , I I I s flames, the condition of ua/uf = 1 is denoted by 0.0 1.0 2.0 3.0 4.0 reverse-shaded s_nbols on the plots (note that the U=(m/s) soot-formation and soot-formation-oxidation config- urations occur for test conditions in the left and right FIG 4. Fuel flow rates at laminar soot-point and liftoff of the reverse-shaded symbols, respectively). Liftoff condibons as afunction of air coflow velocities, fuel-port conditions are denoted by the symbol at the higliest diameter, and pressure for ethylene/air flames. air flow rate for each pressure and fuel-port diame- ter, with the extreme liftoff limit denoted by a dashed line. shape correlations based on the simplified analyses The measurements illustrated in Figs. 3--6 show of Refs. [22] and [23]. Ignoring small effects of vir- that increased air coflow velocities increase laminar tual origins, both these correlations can be written soot-point fuel rates. Notably, this behavior is ob- to yield the luminous flame length as a function of served for air/fuel-stream velocity ratios both the corrected flow rate parameter used in Fig. 2, as smaller and larger than unity. Increasing pressures follows: generally reduce allowable fuel mass flow rates and flame lengths for soot-free flames due to increased L = (C,CfSc/(8zr)) mf/(Zst/2) (1) soot-formation rates and flame residence times for a Following Refs. [22] and [23], a simple correlation gwen flame length. The relative enhancement of of equation 1was fitted to measurements of flames laminar soot-pomt fuel flow rates between small and 71 HYDRODYNASMUPICPRESOSFIOSONOFTORMATION 2089 2.0 i I = I i | w soot-suppression argument discussed in the intro- PRESSURE PROPANE duction. The resulting soot-free flames also provide t_k.lDa,:10.2 SYM. d(mm) potentially useful conditions for evaluating detailed A_E] 0 • 1.6 A/D t D• 3.2 models of diffusion flame chemistry and transport at 1.5 ' /_E) _ _• 4.8 the computationally tractable limit of soot-free lam- .a_ o _ 13.6 ;'ffT_E.ses_o inar diffusion flames for light hydrocarbons. •_ A _'_ SYMBOL DENOTES For the present tests, the propensity of a fuel to soot can be associated with the pressure range for 1.0 .g _, 23.7/--LIFT-OFF LIMI' observing soot-free flames. On this basis, the present tests indicate that the propensity to form and emit soot progressively decreases in the order acetylene, _,',. AP_,. _ I" ,_ 33.9 ethylene, propane, and methane. This finding agrees 0.S with conventional determinations of laminar smoke- point properties based on observations of buoyant laminar jet diffusion flames [4--8]. In addition, the general behavior of the laminar soot-point properties 0.0 I I = I = I = 0.0 0.4 0.8 1.2 6 in Figs. 3--6 is qualitatively similar to earlier obser- vations of laminar smoke-point properties as afunc- u. (m/s) taon of air coflow velocities in Ref. [16]. FIG. 5. Fuel flow ratesatlaminar soot-pointand|iftoff An important issue concerning the results illus- conditions as a function of air coflow velocities, fuel-port trated in Figs. 3--6 is the mechanism for increased diameter, and pressure forpropane/mr flames. resistance to soot formation as the air coflow velocity increases for a particular fuel, fuel-port diameter, and pressure. Consider the simplest case, wlien the 3.0 ' = I ) I i I = flame is in the soot-formation-oxidation condition for I •ETHANE l sY. (m air/fuel-stream veloc]ty ratios greater than unity, r bi ,.6 which generally involves conditions in which buoy- / PRESSURE(kPa): o • 3.2 ancy does not significantly affect flame velocities. / , 22.0 A • 4.8 The results discussed in connection with Fig. 2 then 20r indicate that the flame shape (length) is largely con- t ultu f :1 trolled by the fuel flow rate and is relatively inde- pendent of fuel velocity at the burner exit (or the g .E- burner-port diameter). In contrast, the characteristic flame residence time, t_,is proportional to the flame length divided by the mr coflow velocity [23]. Thus, 1.0 i _ m _....._._11 49,8 given acritical residence time for the appearance of soot for a particular fuel and pressure, the fuel flow rate at the laminar soot-point limit progressively in- creases with increasing air coflow velocity, relatively 0.0 I I I I I I I I independent of fuel-port diameter, which is typical 0.0 0.4 0.8 1.2 1.6 of the behavior seen in Figs. 3-6 for reasonably large u,(m/s) air/fuel-stream velocity ratios. The mechanism of increased resistance to soot for- FIG 6.Fuel flow rates atlaminar soot-point and hftoff mation as the air eoflow velocity increases for apar- conditions as afunction of air coflow velocities, fuel-port ticular fuel, fuel-port diameter, and pressure ismore diameter, and pressure for methane/air flames. complex when the flame is in the soot-formation configuration (at least near the flame base). This maximum allowable values of air coflow velocities generally involves conditions in which buoyancy af- before liftoff, however, tends to be relatively inde- fects flame velocities and air/fuel-stream velocity ra- pendent of the pressure for a particular fuel. This tios are less than unity. For such conditions, increas- behavior comes about because generally more in- ing the air coflow velocity causes the flame to shift tense reaction rates at elevated pressures accom- from the soot-fonnation toward the soot-formation- modate large air coflow velocities before liftoff, oxidation configuration, which reduces the propor- which tends to compensate for faster soot reaction tion of the flame residence time spent at soot-for- rates at elevated pressures. Taken together, it is clear mation condihons compared with soot-oxidation that sufficiently large air eoflow velocities are capa- conditions and thus tendencies for soot formation. ble of completely suppressing the formation of par- Behavior of this nature can be observed from the ticulate soot for these conditions, supporting the soot-concentration measurements near laminar 72 9,090 LAMINADRIFFUSIOFLNAMES---CJoefFltolawmes smoke-pocinotnditioninsRef.[16]w, herevaria- that laminar smoke-point flame lengths in both tionsofsooctoncentratiaosnasfunctioonfresi- coflowing and still air environments are roughly dencteimebecompeathindependeanstthesoot- twice as long as soot-free (blue) flames under formation-oxidatcioonnditionis approached. comparable conditions due to the presence of lu- Similarltyh,iseffecitsnotuniformforallsooptre- minous soot particles under fuel-lean conditions cursoprathtshrougthhepresefnlatmews,hereaasll as laminar smoke-point conditions are ap- pathsareaffectetdosomeextenbtyreducefldame proached. residentcimeesasaircoflovwelocitieasreincreased. The mechanism of increased resistance to soot Theseeffectsa,ndtheintrusioonfbuoyanicnyt,ro- 3. formation with increasing air/fuel-stream velocity ducegreateerffectosffuel-podritametoenrlaminar ratios at low pressures (where buoyancy does not soot-pocinotnditiofnosrthesfelamefosrthesimple significantly affect flame velocities) and large air/ soot-formation-oxidflaatmioenconfiguratidoins- fuel-stream velocity ratios (where the flame is in cusseedarliear,sseeinnFigs3.--6N.evertheleinss, the soot-formation-oxidation configuration) in- spiteofvariationosfflamebehavidoerpendionng therangoefair/fuel-strevaemlocitryatiosandef- volves progressive reduction of flame residence fectsoftheintrusioonfbuoyancthye,generacal - times for soot production, eventually reaching the soot-free (blue) flame limit. Given a critical resi- pabilitoyfincreaseadircoflowvelocitietosreduce thecontenatndemissioonfssooftorthepresent dence time for the appearance of soot for a par- flameissevident. ticular fuel and pressure, this behavior is consis- tent with present measurements and the simplified analysis of the shape of non-buoyant Flame Stability Properties laminar jet diffusion flames in coflowing air [23]. Notably, the shape (length) of these flames is Limiting conditions for fame liftoff are plotted in largely controlled by the fuel flow rate, while the Figs. 3-6 as a function of pressure for each fuel. At characteristac residence time is proportional to high pressures, fuel-port velocities are small atliftoff the flame length divided by the air coflow veloc- conditions, and this limit correlates quite nicely as a ity. Then, laminar soot-point fuel flow rates function of coflow velocity and pressure, relatively should increase with increasing air coflow veloc- independent of fuel-port diameter. At low pressures, ities for a given fuel and pressure, relatively in- however, fuel-port velocities become relatively large dependent of fuel-port diameter, as observed at and also begin to affect liftoff conditions, with small low pressures and large air coflow velocities in fuel-port diameters (wbich yield the largest fuel-port Figs. 3--6. velocitles) generally contributing to reduced flame stability. 4. The mechanism of increased resistance to soot formation with increasing air/fuel-stream velocity ratios is more complex at high pressures (where Conclusions buoyancy significantly affects flame velocities) and atsmall air/fuel-stream velocity ratios (where The present experimental investigation consid- the flame is in the soot-formation configuration). ered the effect of air/fuel-stream velocity ratios on Then, increasing air/fuel-stream velocity ratios soot processes within laminar coflowing jet diffusion causes the flame to shift from the soot-formation fames for the experimental conditions summarized toward the soot-formation-oxidation configura- earlier. Major conclusions of the study are as follows: tion, which reduces the proportion of the flame 1. Laminar soot-point flame lengths and fuel flow residence time spent atsoot-formation conditions rates were increased with increasing air/fuel- compared with soot-oxidation conditions, reduc- stream velocity ratios; these effects were most ing tendencies for soot formation accordingly. pronounced at low pressures, where effects of However, this effect is not uniform for all soot buoyancy were minimized, and mitial air/fuel- precursor paths through the flame, whereas all stream velocity ratios are reasonably representa- paths are affected to some degree by reduced twe of the entire visible portion of the flame for flame residence times with increasing air/fuel- the present test conditions. These results are stream velocity ratios, as discussed in conclusion qualitatively similar to earlier measurements of 3 above. lammar smoke-point properties, as well as recent predictions of soot-concentration properties [17], Other effects observed during the present inves- for similar flame conditions. tagation generally are consistent with earlier findings 2. Laminar soot-point flame lengths were conven- concerning the propensity of diffusion flames to iently correlated in terms of acorrected fuel flow form and emit soot [7-8]: laminar soot-point fuel rate parameter based on an earlier simplified flow rates and flame lengths tend to progressively analysis of the structure of non-buoyant laminar increase with decreasing pressure, and the propen- coflowmg jet diffusion flames [23]. It was found sity to form and emit soot vath variations of fuel type 73 HYDRODYNAMIC SUPPRESSION OF SOOT FORMATION 2091 progressively decreases in the order acetylene, eth- 4. Schalla, R. L., and McDonald, G. E., Proc. Combust ylene, propane, and methane. Finally, inspite oflim- Inst. 5:316--,324 (1954). itations due to the intrusion of buoyancy, the results 5. Schug, K. P., Manheimer-Timnat, Y.,Yaccarino, P.,and of the present investigation support the earlier find- Glassman, I., Combust. Sci Technol. 22:235-250 ings of Ref. [16] that effects of enhanced air/fuel- (1980). stream velocity ratios contribute to the mechanism 6. Flower, W. L., and Bowman, C T., Proc Combust of reduced sooting tendencies for non-premixed Inst. 21:1115-1124 (1983). flames using air atomization techniques. Neverthe- 7. Sunderland, P. B., Mortazavi, S., Faeth, G. M., and less, more work isneeded to resolve the specific con- Urban, D. L., Combust, Flame 96:97-103 (1994). tributions of enhanced air/fuel-stream velocity ratios 8. Urban, D. L., Yuan, Z.-G., Sunderland, P. B., Linteris, and improved atomizalaon to reducing the sooting G. T., Voss, J. E., Lin, K.-C., Dai, Z., Sun, K., and tendencies of practical spray flames. Faeth, G. M, AIAAJ 36-1346-1360 (1998). 9. Sugiyama, G., Proc. Combust lnst 25:601-608 (1994). Nomendature 10. Du, J., Axelbaum, R. L., and Law, C K., Proc. Corn- bust. Inst. 22:387-394 (1988). Cf flame length empirical parameter 11. Du, J., and Axelbaum, R. L., Combust. Flame 100:367- C, flame length configuration parameter 375 (1995). d fuel-port diameter 12. Sunderland, P. B., Axelbaum, R. L., and Urban, D. L., D mass diffusivity in Fifth International Mtcrogravity Combustion Work- Era, Frf air- and fuel-stream Froude numbers, shop, report NASA/CP-1999-208917, NASA, Wash- (ua2or u_)/(2gL) ington, DC, 1999, pp. 475-478. g acceleration of gravity 13. Kang, K. T.. Hwang, J. Y, Chung, S. M., and Lee, W., L laminar smoke- and soot-point flame Combust. Flame 109:266-281 (1997). lengths 14. Lin, K.-C., and Faeth, G M., J. Prop. Power 12:691- mf fuel mass flow rate 698 (1996). P pressure 15. Lm, K.-C., and Faeth, G. M, Combust Flame Re Reynolds number, 4 7h/(nd/a) 115:468-480 (1998). Sc Schmidt number, v/D 16. Lm, K -C., and Faeth, G. M.,J Prop Power 12.10--17 tt characteristic residence time, L/ua (1996). U streamwise velocity 17 Kaplan, C R., and Kailasanatb, K, Combust Flame, Zst stoichiometric mixture fraction in press (2000). /1 dynamic viscoslty 18. Markatou, P, Wang, H., and Frenklach, M., Combust. Y kinematic viscosity Flame 93.467--482 (1993) Subscripts 19. Sun, C J., Sung, C. j, Wang, H., and Law, C. K., a imtial property of airstream Combust Flame 107:321-335 (1996). f initial property of fuel stream 20. Lm, K-C., and Faeth, G. M., Envtron. Combust Tech- nol. 1:53 (2000). Acknowledgments 21. Law, C K., and Faeth, G M., Prog Energy Combust Sc/. 20.65-113 (1994). Thts research was supported by NASA grants NCC3- 22 Lin, K.-C., Faeth, G. M., Sunderland, P B, Urban, 661, NAG3-1878, and NAG3-2048 under the techmcal D. L., and Yuan. Z. G., Combust. Flame 116415--431 management of D. L. Urban and Z-G. Yuan of the NASA (1999) Glenn Research Center. 23 Lin, K-C., and Faeth, G M., AIAA J. 37 759-765 (1999). 24 Spalding, D B., m Co,d)ustion and Mass Transfer, REFERENCES Pergamon Press, New York, 1979, pp. 185-195. 25. Schlichtmg, H., Boundary Layer Theory, 4th ed ,Mc- 1 Balm D. W., in Gas Turbine Combustion Design Prob- Graw-Hill, New York, 1960, pp. 169-164. lems (A H. Lefevre, ed.), Hemisphere, Washington. 26. Mahahngam, S., Fer'zlger, J H., and Cantwell, B. J., DC, 1979, pp. 205-223 Comkntst Flame 82 231-234 (1990). 2 Hussman, A. W., and Maybach, G. W., SAE Trans 27. Sunderland, P. B., Koyhi, I). O., and Faeth, G. M., 69:563--574 (1961). Combust. Flame 100.310--322 (1995). 3. Hayaaes, B S., and Wagner, H. G., Prog. Energy Corn- 28. Sunderland, P. B., and Faeth, G M., Combust Flame bust Sci. 7:229-273 (1981). 105.132-146 (1996). 74 2092 LAMINAR DIFFUSION FLAMES---Coflow Jet Flames COMMENTS C. H. Priddin, Rolls Royce, UI( In the 80s-style fuel Cary Presser, NIST, USA. Please describe your thoughts atomizers you showed, the overall AFRs are of the order regarding the use of different gases in place of air. Is the 4-6, that is, still overall rich. Do you think your analysis propensity to soot purely an aerodynamic effect (and thus still applies in this situataon, or is the flame somewhere other gases may be used) or isthe pressure of oxygen re- else? quired to assist in the oxidation of soot? It is assumed that ambient (or secondary) air is present to sustain a stable Author's Reply The general success of air atomization flame. to reduce soot emissions from aircraft gas turbine enganes for a variety of fuel atomizer AFRs [Ref. 1in paper] sug- gests that effects of increasing art/fuel velocity ratios persist Author's Reply For the same reasons discussed in the even when AFRs are small. We believe that this isreason- reply to C. H. Pndden, we believe that the nature of the able based on present findings because small fuel stream atomizing gas used in the fuel atomizer is not the most velocities should generally provide conditaons where air critical aspect of soot control using air atomization. It stream velocities are larger than fuel stream velocities seems to us that the crucial elements are relatively good throughout the combustaon process, leading to generally atomization with relatively small fuel momentum (veloci- desirable soot emissions properties, e.g., soot-formation- ties). This should generally yield desirable air/fuel stream oxidation conditions as defined by Kang [Ref. 13 in paper]. velocity ratio properties when the region of the flame sheet Direct demonstration of this conjecture, however, would is approached, e.g., soot-formation-oxadafion eonditaons as be desirable. defined by Kang (Ref. [13] in paper). Direct assessment of the conjecture, however, would also be desirable.
