Larvae of Abachrysa eureka (Banks) (Neuroptera: Chrysopidae: Belonopterygini): descriptions and a discussion of the evolution of myrmecophily in Chrysopidae PDF
Preview Larvae of Abachrysa eureka (Banks) (Neuroptera: Chrysopidae: Belonopterygini): descriptions and a discussion of the evolution of myrmecophily in Chrysopidae
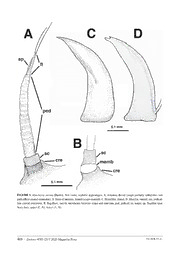
Zootaxa 4789 (2): 481–507 ISSN 1175-5326 (print edition) Article ZOOTAXA https://www.mapress.com/j/zt/ Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4789.2.7 http://zoobank.org/urn:lsid:zoobank.org:pub:1DF30B06-84E1-447C-8E71-4B91EB52AB32 Larvae of Abachrysa eureka (Banks) (Neuroptera: Chrysopidae: Belonopterygini): descriptions and a discussion of the evolution of myrmecophily in Chrysopidae CATHERINE A. TAUBER1*, SHELBY KERRIN KILPATRICK2,3,4 & JOHN D. OSWALD2,5 1Department of Entomology, Comstock Hall, Cornell University, Ithaca, NY, USA 14853 and Department of Entomology and Nematol- ogy, University of California, Davis, CA, USA 95616. (cid:1)[email protected]; https://orcid.org/0000-0003-1310-8996 2Department of Entomology, Texas A&M University, College Station, TX, USA 77843 3Current Address: Department of Entomology, Pennsylvania State University, University Park, PA, USA 16802 4(cid:1)[email protected]; https://orcid.org/0000-0003-2760-1722 5(cid:1)[email protected]; https://orcid.org/0000-0001-7977-9309 *Corresponding author Abstract Here we describe the first and third instars and the egg of the New World chrysopid Abachrysa eureka (Banks). Like other members of the tribe Belonopterygini, this species is myrmecophilic. Comparisons of Abachrysa larval and egg characteristics with those reported from four other belonopterygine genera indicate that Abachrysa more closely resembles the Old World Calochrysa and Italochrysa than the New World Vieira and Nacarina. The three genera Abachrysa, Calochrysa and Italochrysa all have large eggs, accelerated embryonic development, and an elaborate set of morphological modifications for larval debris carrying, an important defense against ant attack. This pattern of shared features is consistent with the phylogenies recovered in recent molecular studies that place the New World genera Vieira and Nacarina basal to Abachrysa and the Old World genera. Our assessment of current morphological information in relation to the molecular studies indicates the following sequence: (i) The form of belonopterygine myrmecophily that is currently expressed in the basal lineages (Nacarina and perhaps Vieira) originated in the New World and does not involve elaborate larval modifications for debris carrying. (ii) Myrmecophily that is based on a correlated set of developmental and morphological traits subserving debris carrying evolved in the New World when Abachrysa diverged. (iii) Subsequently, the debris-carrying clade of Belonopterygini underwent a significant radiation in the Old World, but not in the New World. Key words: Debris carrying, egg size, embryonic development, phylogeny, geographic distribution Dedication We dedicate this article to the memory of Ellis G. MacLeod (1928–1997, University of Illinois), a devoted educa- tor and student of Neuroptera. In 1968, his student Joseph Sheldon collected a gravid female of Abachrysa eureka (Banks) and sent it to Ellis for rearing. Ellis was successful and today remains as the first and only person known to have reared a belonopterygine chrysopid from egg to adult. However, he never published the results of that work. Upon his death his fine drawing and photographs of the A. eureka egg and third instar, along with his notes and an early draft of a manuscript on this species, passed to JDO at Texas A&M University. His results are now included here with our findings on the first instar and egg. Introduction The cosmopolitan Belonopterygini is one of five tribes now included in the green lacewing subfamily Chrysopinae (Garzón-Orduña et al. 2019, Winterton et al. 2019). This tribe has several distinctive features, the most interesting of which is its reported use of ant brood as prey, a feature that has not been associated with any other group of green Accepted by A. Letardi: 20 Apr. 2020; published: 9 Jun. 2020 481 Licensed under a Creative Commons Attribution 4.0 International License http://creativecommons.org/licenses/by/4.0/ lacewings. Belonopterygini is a relatively small tribe; currently, it consists of 15 genera. Four of these are known from the New World (North and South America), and eleven occur in the Old World (loosely defined here as Europe, Asia, Africa, and Australia) (Brooks & Barnard 1990, Tauber et al. 2006, Tauber 2007, Winterton & Brooks 2015, Sosa & Tauber 2017, Oswald 2019). Belonopterygine larvae are rarely encountered in the field. Indeed, until this report the mature larvae of only two species were described. Both were found in association with ant nests, and both are from the genus Italochrysa: the European Italochrysa italica (Rossi) described by Principi (1943, 1944, as Nothochrysa italica Rossi) and the Australian Italochrysa insignis (Walker) described by Tauber & Winterton (2014). In addition, larval associations with ants were reported for two New World belonopterygine genera: Nacarina and Abachrysa (Weber 1942, Ma- cLeod’s unpublished notes). Unfortunately, specimens associated with these two reports are not known. Field-collected belonopterygine females occasionally oviposit when they are held in vials or cages; conse- quently, over the years, first instars from three genera have become available for morphological study. As demon- strated by Tauber et al. (2006, 2014) and Monserrat & Díaz-Aranda (2012), the comparative morphology of first instar chrysopids can provide considerable systematic and phylogenetic information. The initial descriptions of belonopterygine first instars came from New (1983, 1986): I. insignis and Calochrysa extranea (Esben-Petersen); Díaz-Aranda & Monserrat (1995): Italochrysa stigmatica (Rambur); and Tsukaguchi (1995): Italochrysa japonica (McLachlan). Subsequently, Tauber et al. (2006) described the first instar of Vieira elegans (Guérin-Méneville) [as Berchmansus elegans (Guérin-Méneville)], and Monserrat & Díaz-Aranda (2012) added detailed information for I. stigmatica. Here, we describe and present comparative data from the first and third instars of the New World Abachrysa eu- reka (Banks). The information on the first instar is derived from specimens obtained by the second and third authors (SKK, JDO), and that on the third instar stems from MacLeod’s drawing, photos, and notes. In addition to descrip- tions, our report evaluates the consistency of larval features previously proposed to be synapomorphic for the tribe Belonopterygini, and it discusses the evolution of belonopterygine myrmecophily within the context of the newly available data on Abachrysa and recently published phylogenetic studies of chrysopid genera (Garzón-Orduña et al. 2019, Winterton et al. 2019). Materials and methods Specimens. Adult specimens were collected between 15 August and 17 October 2015 at two localities in College Station, Brazos County, Texas, U.S.A. (30.58849°N, 96.25366°W and 30.53145°N, 96.28170°W ± 120m [WGS 84]). Collections were primarily made at sheets lit with black lights or mercury vapor bulbs, and by searching the surrounding area during the evening and early the following morning. Adults were held in plastic vials containing a paper strip as an oviposition site. After oviposition, eggs and first instars were held in vials and offered food under laboratory conditions. Some of these larvae were preserved for the morphological study here. Other larvae were randomly selected for feeding trials and offered a variety of prey as food; none of the first instars fed sufficiently to molt to the next instar. First instars selected for morphological study were boiled in water for about one minute and then placed in 80% EtOH. Two days later, some (n=19) were transferred to 10% KOH for clearing. After another two days, ten of these larvae were transferred to chlorazol black for staining. After being dehydrated through increasing concentrations of EtOH, all 19 larvae were slide mounted in Canada balsam under coverslips. Additional first instars (n = 48) were point mounted, as were unhatched eggs (n = 8) and eclosed chorions (n = 30); their stalks were included when pos- sible. All of these specimens, as well as the field-collected adults, were deposited in the Texas A&M University In- sect Collection (TAMUIC) under Voucher Number 712. Several larval specimens (n = 4 on slides, 4 point mounted) were retained in the Tauber research collection in Davis, CA, for future study. MacLeod’s original drawing, photos, and notes are also held in the TAMUIC. Descriptive procedures and measurements. The description of the first instar was made from both cleared and dried specimens. Slide-mounted specimens (n = 3–4 per structure) were used to estimate the length of the body and to measure the head; because of possible compression, the head measurements (length and width) may be slightly larger than actual size. Thus, caution should be used when comparisons are made with previously published mea- surements by the first author (e.g., Tauber et al. 2006) and others who measured specimens without a coverslip. 482 · Zootaxa 4789 (2) © 2020 Magnolia Press TAUBER ET AL. Measurements included the following: cranial width—across the widest part of the head, between the exterior margins of the eyes; cranial length—along the midline between the anterior and posterior margins of the head; man- dibular length—along the midline of the curved dorsal surface of the mandibles. All measurements were made with Image J public domain software (ver. 1.46r, National Institutes of Health, USA; http://imagej.nih.gov/ij). The description of the third instar is based on MacLeod’s photographs and drawing and is compared with de- scriptions of I. italica by Principi (1943, 1944) and I. insignis by Tauber & Winterton (2014). Nomenclature for larval morphology and setation follows Rousset (1966) for the head, and Stehr (1987), Tauber & de Léon (2001), and Tauber et al. (2006) for the thorax and abdomen. Primary cephalic setae are designated with an “S” followed by a number; setae on the thoracic and abdominal lateral tubercles are designated as LS and LDS respectively, and submedian setae on the dorsum of the thorax and abdomen as SMS. For some taxa or specimens, the taxonomically important thoracic sclerites are easily identified; they are both tanned (light or dark brown, or black) and obviously sclerotized (rigid, smooth, reflective in liquid medium). How- ever, in A. eureka first instars, the entire notum of each thoracic segment (most notably, the pronotum) appeared lightly sclerotized, and we were not able to identify specific thoracic sclerites. Finally, in previous larval descrip- tions by CAT, the integument is described as with or without “spinules”, a term that is only vaguely defined [e.g., Torre-Bueno (1989): spinule, “a small spine (T-B); see microtrichia”], but well illustrated (Stehr 1987: 296). The definition of “microtrichia” stands in contrast to “macrotrichia”, which include hairlike projections that are articu- lated in cuticular sockets. The “spinules” reported for chrysopid larvae appear to be sessile, acute, scalelike, unar- ticulated, and without sockets; thus, they are “microtrichia”. Here we use this term in lieu of “spinules”. To facilitate morphological comparisons, an error in a previous article (Tauber et al. 2006: 222, Fig. 1, as Berch- mansus) is noted here: in this article the basal antennal “segment” labeled as the scape (“sc”) is actually a pedicellate extension of the cranium beneath the antenna, not a segment of the antenna. Thus, the true scape was misidentified as the pedicel, and the true pedicel was misidentified as an enlarged flagellum. The true scape is a simple, unmodi- fied segment; it can be partially withdrawn into the pedicellate cranial extension. The true pedicel is elongate, swol- len, and annulated throughout; it has a very small seta about 1/3 the distance from its tip (not shown in the original figure). The true flagellum, which extends from the tip of the true pedicel, is slender, tapered, and also annulated; it bears a large basal spur (perhaps a modified seta) that extends distally beyond the tip of the flagellum; the tip of the flagellum bears a pair of slender, elongate terminal setae. These corrections are illustrated in Fig. 14B. Descriptions of the Immature Stages of Abachrysa eureka (Banks) Egg (Fig. 1) Length 3.0 mm, width 1.1 mm (n = 1); fusiform, with apical pole bearing micropyle; laid singly on an erect, smooth, shiny stalk. Stalk 9.2 mm long (n = 1); sticky, but without fluid droplets. Catanach (2007) provided additional mea- surements (see Table 1). Catanach (2007) stated that eggs were pale blue-gray if fertile, but light green if infertile. Subsequent work has shown that eggs are light green when laid; if fertile and healthy, they assume a pale bluish-gray color as the embryo becomes visible beneath the chorion. First instar (Figs 2–9) Body. Small, compact, slightly thickened dorsoventrally through mesothorax, metathorax, and anterior abdominal segments, but dorsal surface probably not abruptly elevated; length of shriveled, unfed specimens slightly greater than 1.5 mm. Integument smooth, without microtrichia, bearing four types of setae: (i) moderately long to medium length, stout, slightly denticulate, with acute tip (primary cephalic setae); (ii) long, robust, lightly denticulate to smooth, straight-to-curved basally, curved-to-bent distally, with acute apex (most setae on the lateral and laterodor- sal tubercles of the thorax and abdomen; LS, LDS); (iii) very long, slender, smooth, curved submedian setae (SMS) on dorsum of mesothorax, metathorax, and first to sixth abdominal segments; (iv) short to medium length, straight, smooth, with acute tip (some primary setae on the cranium, pronotum, seventh and eighth abdominal segments). The SMS are extremely tapered and thin distally; it is difficult to determine if the tips of these setae are acute or minutely hooked. ABACHRYSA LARVAE Zootaxa 4789 (2) © 2020 Magnolia Press · 483 FIGURE 1. Abachrysa eureka (Banks), egg (with stalk), lateral. Photo: E. G. MacLeod. 484 · Zootaxa 4789 (2) © 2020 Magnolia Press TAUBER ET AL. FIGURE 2. Abachrysa eureka (Banks), first instar, head and thorax, dorsal (specimen point mounted). A. Dorsal cephalic setae visible. B. Flagellar setae visible. fls flagellar setae; SMS, submedian setae of thorax; T1-LT, T2-LT, T3-LT, lateral tubercles of prothorax, mesothorax, and metathorax. ABACHRYSA LARVAE Zootaxa 4789 (2) © 2020 Magnolia Press · 485 FIGURE 3. Abachrysa eureka (Banks), first instar, head (specimen point mounted). A. Lateral view. B. Ventral view. cls, clypeal setae; co, cardo; cr, cranium; cre, pedicellate cranial extension; eye, eye including six stemmata; fl, flagellum; fls, flagel- lar setae; lp, labial palpus; md, mandible; men, mentum; mx, maxilla; ped, pedicel; pg, palpiger; sc, scape; st, stipes. 486 · Zootaxa 4789 (2) © 2020 Magnolia Press TAUBER ET AL. FIGURE 4. Abachrysa eureka (Banks), first instar, head. A. Dorsal view (tip of right mandible and labial palps excluded). B. Ventral view (antenna, mandible excluded). cly, clypeus; co, cardo; cr, cranium; cre, pedicellate cranial extension; lp1, lp2, lp3, basal, middle, and distal subsegments of labial palpus; md, mandible; mx, maxilla; pg, palpiger; st, stipes; S1–S12, primary cephalic setae; Vx, three short posterior setae surrounding a pore. Cranium (Figs 2–5). Width 0.54–0.55 mm; length ~0.46–0.47 mm. Dorsum smooth, well sclerotized; posterior margin quadrate, partially withdrawn into cervix (larva at rest); anterior region beneath base of antenna forming pedicellate extension, capable of receiving retracted base of scape. Six stemmata, all well separated, relatively small. All primary cephalic setae (S1–S12) present, with acute tips (Figs 4, 5). Dorsal setae (S1–S7, S11, S12) with surface slightly denticulate; S11, S12 robust, long, directed anteriorly; S1, S2, S3, S6 medium length, robust, but slightly more slender than S11, S12; S5 relatively small; Vx with three short setae, pore detected on some speci- mens; anterior region of cranium (anterior to S11) with two pairs of small, smooth, acute setae (possibly S14-cl, S15-cl of V. elegans); anterior tip of clypeus with pair of large, lightly denticulate, acute setae projecting anteriorly (possibly S13-cl of V. elegans). Venter with cardo and stipes narrow, elongate, rectangular; primary setae (S8–S10) smooth, short to medium length; S8 posterior to eye (sometimes near cardo); S9, S10 near each other, medial to eye. Ventral midregion with ~three pairs of setae on or near mentum; base of palpiger with single seta. Cephalic appendages (Figs 4–6). Clypeus large, extending laterally toward base of mandibles; anterior margin extending forward as truncated process. Mandible short, stout, heavily sclerotized, especially at tip and along lateral margin of distal half; 0.35–0.39 mm long; 0.11–0.13 mm wide, at base; with sharply acute tip, ~7–8 acute teeth in saw-like row along inner edge just below apex. Maxilla broad basally, with two short basolateral setae; lateral margin with two acute teeth (spurs) near terminus, basal one small, pointed basally, distal one larger, near terminus, pointed apically; tip rounded, heavily sclerotized, with small patch of microsetae. Labial palp extending to tip of mandible or slightly beyond; second segment broad (~0.05 mm wide at widest point; ~0.10–0.12 mm long), with ABACHRYSA LARVAE Zootaxa 4789 (2) © 2020 Magnolia Press · 487 FIGURE 5. Abachrysa eureka (Banks), first instar, cephalic appendages. A. Antenna, dorsal (scape partially withdrawn into pedicellate cranial extension). B. Base of antenna, lateral (scape exerted). C. Mandible, dorsal. D. Maxilla, ventral. cre, pedicel- late cranial extension; fl, flagellum; memb, membrane between scape and cranium; ped, pedicel; sc, scape; sp, flagellar spur. Scale bars: upper (C, D), lower (A, B). 488 · Zootaxa 4789 (2) © 2020 Magnolia Press TAUBER ET AL. FIGURE 6. Abachrysa eureka (Banks), first instar, habitus, lateral (specimen point mounted). cox, coxa; fem, femur; fl, flagel- lum; fls, two flagellar setae; prn, pronotum; tar, tarsus; tib, tibia; A7, A9, A10, seventh, ninth, and tenth abdominal segments; A4-LT, A6-LT, lateral tubercles of fourth and sixth abdominal segments; T1-LT, T2-LT, T3-LT, lateral tubercles of prothorax, mesothorax and metathorax three to four annulations; terminal (third) segment rounded, tapering distally, terminus with small, pale, round pro- jection bearing ventral pore and several microsetae apically; maximum width of terminal segment approximately one-half maximum width of second segment. Basal (first) palpal segment with two pairs of long distal setae, one lateral, one mesal; terminal annulation of middle segment with two long setae near apex, one lateral, one mesal. Antenna 0.35–0.38 mm long; scape set within pedicellate cranial extension, heavily sclerotized, rounded, tubular, straight sided, with sharp projection on lateral base; pedicel elongate, tapering, with ~five or six rounded annula- tions on basal half, with irregular annulations on distal half. Flagellum round in cross section, narrow, tapering to slender, bifurcated terminus; base with lateral spur; spur sheath-like, slender, elongate, wrapped partially around flagellum, extending distally almost to terminus of flagellum, closely pressed against lateral margin of flagellum (often difficult to see); terminus with two elongate (length up to 0.1 mm), very fine terminal setae extending anteri- orly, then curving toward each other, with mesal seta usually longer than lateral one. Cephalic coloration (Figs 2, 3, 6). Anterodorsal surface of cranium entirely dark brown, becoming pale near posterior margin (normally concealed within cervical membrane), no specific cephalic markings distinguished; integument around and between stemmata dark brown; pedicellate cranial extension dark brown dorsally, pale ven- trally. Venter with cranial margin, sclerites dark brown; intersegmental membrane pale. Antenna with scape, base of pedicel dark brown; pedicel with annulations brown basally, lighter brown distally; flagellum light brown to amber. Mandible and maxilla brown basally, light brown to amber distally. Labial palp with basal segment dark brown; an- nulations of second segment brown, membrane between annulations pale; distal segment brown basally, becoming lighter brown to amber distally. ABACHRYSA LARVAE Zootaxa 4789 (2) © 2020 Magnolia Press · 489 FIGURE 7. Abachrysa eureka (Banks), first instar, thorax, dorsal. A. Prothorax. B. Mesothorax and metathorax (SMS more numerous than shown). LS, setae on lateral tubercle; LT, lateral tubercle; SMS, submedian setae; Sp, spiracle. Scale applies to A and B. 490 · Zootaxa 4789 (2) © 2020 Magnolia Press TAUBER ET AL.
