Immunology of Silicones PDF
Preview Immunology of Silicones
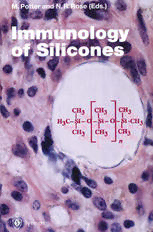
Current Topics in Microbiology 210 and Immunology Editors R.W. Compans, Atlanta/Georgia· M. Cooper, Birmingham/Alabama· H. Koprowski, Philadelphia F. Melchers, Basel· M. Oldstone, La Jolla/California S. Olsnes, Oslo· M. Potter, Bethesda/Maryland H. Saedler, Cologne· PK. Vogt, La Jolla/California H. Wagner, Munich Springer Berlin Heidelberg New York Barcelona Budapest Hong Kong London Milan Paris Santa Clara Singapore Tokyo Immunology of Silicones Edited by M. Potter and N. R. Rose With 136 Figures and 132 Tables • Springer MiCHAEL POTTER, M.D. Chief Laboratory of Genetics Bldg. 37, Rm. 2B04 National Cancer Institute National Institutes of Health 37 Convent Drive MSC 4255 Bethesda, MD 20892-4255 USA NOEL R. ROSE, M.D. PH.D. Professor of Pathology and Molecular Microbiology and Immunology Departments of Pathology and of Molecular Microbiology and Immunology John Hopkins University 615 N. Wolfe Street, Rm. 4027 Baltimore, MD 21205 USA Cover illustration: INFLAMMATORY SILICONE GEL GRANULOMA The early response to sl1icone gels is characterized by a dense cellular infiltration of different cell types. This figure shows stages in the formation of giant cells around the sl1icone gel vacuoles. Cover design: Kiinkel+Lopka, IIvesheim ISBN-13: 978-3-642-85228-2 e-ISBN-13: 978-3-642-85226-8 001: 10.1007/978-3-642-85226-8 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable for prosecution under the German Copyright Law. @ Springer-Verlag Berlin Heidelberg 1996 Softcover reprint of the hardcover 1st edition 1996 Library of Congress Catalog Card Number 15-12910 The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and application contained in this book. In every individual case the user must check such information by consulting other relevant literature. Typesetting: Camera-ready by authors SPIN: 10497576 27/3020 - 5 432 1 0 - Printed on acid-free paper Preface This issue of Current Topics in Microbiology and Immunology records the proceedings of a Workshop on the Immunology of Sili cones held at the Natcher Conference Center, National Institutes of Health, Bethesda, Maryland, March 13 and 14, 1995. A large num ber of investigators from North America and Europe met to discuss available data on how the immune system responds to silicones and related materials. Some aspects of this field are controversial. Nonetheless, the meeting was marked by a civil and open ex change of scientific information and divergent interpretations, re flecting the traditions of scientific communication. Each invited participant was asked to submit an article sum marizing his/her presentation. Most of the papers are published as submitted, with only editorial changes to conform with the guide lines given to each contributor or revisions to clarify aspects of the paper. The papers should not be regarded as peer-reviewed publi cations. This preface will attempt to outline some of the immu nological areas of investigation relating to silicones. GENERAL The great range of viscosity of liquid and cross-linked silicone poly mers and the elasticity of silicone rubbers made it possible to fabricate materials that mimic the consistency of tissues ranging from bone to mammary tissue. This has brought silicones into wide usage, and probably the largest group of persons involved are the recipients of silicone gel-filled breast implants (SBls). It has been estimated that at least 800,000 women in the U.S. alone have been implanted with SBls over the last two decades [see COOK]. Im plants are not stable indefinitely, and after a period of years sil icone materials will ultimately leak into tissues. An issue of considerable controversy is whether implants evoke disease. Although the issue of silicone-related disease remains a major clinical concern, it was not the primary subject of this meeting, rather the discussions focused on evidence about how the immune system responds to silicones. VI Preface CHEMISTRY OF SILICONES Silicones are synthetic chemicals that do not occur naturally, thus the Si-O backbones in these long polymers are not directly (if at all) attacked by the enzymatic machinery of the body. The commonly used mammary implants contain two physically different forms of silicone polymers, the highly crosslinked elastomeric shell or casing (elastomer) and the silicone gel contained within. Both contain multiple molecular species of silicones. Elastomer The elastomer casing is made of highly crosslinked silicones, and also contains fumed silica. It is not impermeable, and liquid sili cones within can slowly escape. The elastomer interfaces with connective tissues. There is probably a considerable quantitative range of tissue reactions to the elastomer in a genetically hetero geneous population, reflected in the extent of interfacial fibrous capsule formation [SHANKLIN et al.J. Such reactions are not specific for silicone elastomers but might be induced by other polymers which have a similar consistency. As pointed out by WOLF et al., all materials 'age', and silicone elastomers are no exception. Gradually with time (averaging 10 years or more), the elastomer casing may erode .and permit silicone gel to enter the surrounding fibrous tissue capsule and tissue beyond. Because of the presence of fumed silica in the elastomer, it is relevant to discuss the immunology of Si0 (silica). It has been 2 known for many years that certain forms of Si0 are strongly fibro 2 genic [see ALLISON]. This initiates a process that involves the ingestion of the silica particle by macrophages which then secrete fibrogenic cytokines, e.g., TNF-a, TGF-I3. Components of the elastomer shell then may stimulate an inflammatory response via macrophages. Silicone Gel To produce the desired viscosity, the silicone gel is only partially crosslinked and much of the 'gel' is composed of filler, i.e., long chain linear polymers usually 1000 centistoke (cs) polydimethyl siloxane (PDMS). Gels also contain lower molecular weight un polymerized silicones that are derived from the polymerization reactions [see LANE and BURNS). The latter include a series of linear and cyclic compounds ranging from 3 to 300+ siloxy units (1000 cs PDMS has about 330 siloxy units). A frequently discussed low molecular weight compound is octamethylcyclotetrasiloxane which is called D4. The crosslinking step is carried out inside the elastomeric casing. Some silicone gel material, including liquids es cape through the elastomer shell may migrate in tissues and may even be transported to regional lymph nodes. Preface VII A critical question is whether silicone materials in the body can be degraded and give rise to new silicone - related species of molecules, or even silica. Chemical changes also can hypothe tically take place in the gel itself [see BATICH et a/., WOLF et a/.J with the formation of degradation products such as silanols. Only fragmentary data are available on the chemistry and kinetics of this degradation process and on the reactivity of these putative silanols. The fate of low molecular weight silicones with 3 to 10 siloxy units is also not known. There is some early evidence that certain low molecular weight siloxanes are toxic to cells in tissue cultures [FELIX et a/.]. GARRIDO et at. discussed their evidence obtained by nuclear magnetic resonance spectroscopy that the silicone gel itself can be degraded in vivo into products that appear in blood plasma. The chemical identity of such intermediate compounds awaits further analytical data. This area of investigation is not clearly defined, and many more data are essential to determine if biologically active materials are formed from the degradation of the high molecular weight polymers. REACTIVE CAPSULES AND T-CELLS The character and generation of capsules vary with the individual, but, importantly, in some persons the capsule may also contain cells of immune system origin, e.g., lymphocytes, plasma cells and macrophages [see SHANKLIN et a/., HARDT et a/., YOUNG et a/., O'HANLEN et al.]. These interactive tissues may show varying de grees of inflammation. The capsule around a mammary implant is not just a simple fibrous wall between implant and host, but also a tissue buffer zone into which some of liquid materials from the im plant gel contents can enter and become organized as silicone granuloma tissue. These are chiefly liquids that are loosely in corporated in the silicone gel itself during its preparation that slowly bleed through the elastomeric shell. It is difficult to directly quantitate silicones in tissues by chemical methods, but this can be achieved indirectly by oxidizing silicones to silicon. Using this approach PETERS et at. have shown the capsules around implants contain high amounts of extractable silicon. An important aspect of immunological reactivity to SBls con cerns the role of T-Iymphocytes. Silica has been implicated as a possible proximal agonist from the studies of SHANKLIN et at. who have observed Si02 crystals in capsules. They have provided in direct evidence that peripheral blood lymphocytes from SBI recipi ents are hyperreactive in in vitro cell proliferation assays to Si0 2 [SHANKLIN, SMALLEY et a/.J. Further, anti I L-2 inhibited the incor poration of [3HJ thymidine into DNA of Si02 - stimulated lym phocytes. More direct evidence of specific T-cell activity in cap sules is provided in the immunohistopathological studies of VIII Preface O'HANLON et al. They typed the TCR V-gene families expressed in capsular tissues and suggest local immune responses occur in cap sules. YOUNG et al. HLA typed and found a statistically significant overrepresentation of HLA-DR53 in symptomatic patients. In the course of this study they discovered the intriguing finding that 81 % of their symptomatic patients had autoantibodies to B-cells. McDoNALD et al. have provided additional evidence that the intra peritoneal injection of silicone gels in selected strains of inbred mice primes peritoneal cells to induce proliferation in splenic T-cells. WILSON and MUNSON found a suppression in N K cell activity follow ing subcutaneous implantation of silicone gels. ADJUVANCY One of the important questions discussed at the workshop con cerns the immunological adjuvancy of silicones and the specific conditions under which silicones can act as adjuvants. An early publication by Naim, Lanzafame and Van Oss (lmmunol. Invest. 22:151, 1993) indicated that the in vitro emulsification of silicone gels with aqueous solutions of bovine serum albumin (BSA) pro vides miXtures that give a strong enhancement of the humoral im mune responses to BSA in rats even exceeding those obtained with the classical complete Freund's adjuvants (CFA). An important finding in the study by Naim was the striking difference between silicone oils and gels. The (20 cs PDMS) silicone oil was inactive. In a follow-up study (Naim et al. Immunol. Invest. 24:537, 1995), sili cone oils of higher viscosity were tested (100, 350, 1000 and 12,500 cs). The high viscosity (12,500 cs) oil gave a significant in crease in the humoral response 79-98 days later, approaching that of the gel, suggesting increased viscosity and polymer chain lengths are critical factors. The adjuvant properties of silicone gels have been confirmed in other laboratories [see KLYKKEN and WHITE, NICHOLSON et al.l. Octamethylcyclotetrasiloxane (D4), a lower molecular weight silicone precursor found in gels, was also found to have an adjuvant effect [NICHOLSON et al., KLYKKEN and WHITE). This was ascribed to its inflammatory properties. One of the conventional functions of some immunological adjuvants is to capture and slowly release protein antigen. This provides an artificial but nonetheless persistent source of antigen for antigen processing and presenting cells, and sustains immune responses. The high molecular weight silicone oils and gels may vary in efficiency in this respect. While the experiments with silicone gel/protein mixtures provide convincing evidence of adjuvancy when subjected to a manipulated in vitro emulsification procedure, they do not demonstrate that silicone gels which contact tissues produce a similar effect in vivo, particularly in subcutaneous sites. It is premature to draw such a conclusion at this time. Preface IX ASPECIFIC BINDING OF SILICONES TO IMMUNOGLOBULINS AND PROTEINS AND THE QUESTION OF ANTIBODIES TO SILICONE ass VAN and NAIM have discussed and defined the nature of aspecific binding of proteins, in particular immunoglobins (Ig), to hydrophobic surfaces, such as provided by silicones, and showed that IgG in aqueous solution binds to silicone with a high binding energy. They point out that hydrophobic molecules, e.g., silicones "do not fear or repel water. They attract water molecules with considerable energy". Thus, an Ig molecule can bind to a silicone surface via almost any of its solvent surfaces. This property complicates and obscures "the emergence of any paratope that is specific for a given low-energy epitope". In view of these physio chemical considerations, it is not surprising that most studies have failed to identify silicone-specific antibodies [see ROSENAU et al., BUTLER et al., ROSE et al.J. AUTOANTIBODIES The adsorption of proteins to silicone surfaces may denature the protein and hypothetically generate protein-silicone particles that can be taken up by antigen-processing and -presenting cells. The silicones involved in particle formation may be derived from the silicones that bleed through the elastomer shell or from silicone gel materials that escape through larger tears into the subcutaneous spaces. Such immunogenic particles have not been made, but frag mentary evidence suggests that silicone gel material may be more effective than oils [see NAIM et al., POTIER et al.J. The likely avail able antigens would include proteins in connective tissues, tissue breakdown products such as cell membranes, or proteins that are found in inflammatory tissues, e.g., collagens, fibrinogen, fi bronective components of cell membranes (e.g., glycolipids), and cytoplasmic or nuclear nucleoproteins [see KOSSOVSKY et al., ROWLEY et al., ALVING et a/.]. Many of the clinical studies depend upon comparing levels of autoantibodies to such tissue protein in symptomatic versus asymptomatic SBI recipients. Because the symptomatic SBI recipients may have other independently arising conditions, a cause-and-effect relationship between the presence of silicones and antibodies of various specificities cannot be proven. Here, animal studies could be of considerable help. In this connection it is of interest that the i.p. injection of silicone oils (1000 cS DMPS) or silicone gels in mice is associated with the appearance of antibodies to cholesterol [ALVING et al.). The intra peritoneal and subcutaneous sites, however, are very different, as peristalsis and the movement of the intestines could potentially have a physical emulsifying effect. Further, the peritoneal space is associated with a large resident macrophage population. X Preface It is known from independent studies on natural antibodies that many of these are polyreactive with binding activity for chemi cally diverse antigenic specificities, including a variety of autoanti gens [see CAsALI and SCHETIINOJ. These antibodies may arise in an independent way that does not require obvious specific antigenic stimulation. The precursor cells that give rise to these antibodies may be non-specifically stimulated by the presence of silicones. Further research in this intriguing immunological problem may be useful in understanding the immunology of silicones. ANTINUCLEAR ANTIBODIES IN SBI RECIPIENTS Antinuclear antibodies (ANAs) in SBI recipients have been exten sively utilized in clinical studies [CLAMAN and ROBERTSON, BRIDGES et al., SILVERMAN et al., TAN et al., and LEWYJ using the HEp-2 assay to detect ANAs. The range of positive reactions in healthy controls varied from 0-8 %; in healthy SBI recipients, 3-35 %; in symp tomatic SBI recipients, 30-35 %; and in fibromyalgia patients, 25-28 % [CLAMAN and ROBERTSON, BRIDGES et al., SILVERMAN et al.). These data indicate that antinuclear antibody titers are increased in women with SBls. It remains to be established whether the specificity of these antibodies to various nucleoproteins differs in S81 individuals compared with patients with idiopathic autoium mune disease. Differences in the specificities of antibodies to collagen have been described [ROWLEY et al.). Further work in this area is needed to determine if there is a unique profile of autoantibodies in S81 recipients. SILICONE GELS IN MOUSE PLASMACYTOMA FORMATION The Lp. injection of silicone gels, like paraffin oils, triggers or sets in motion the formation of plasma cell tumors in the BALB/c strain of mice that is genetically susceptible to peritoneal plasmacytoma induction. In contrast, the high viscosity 1000 cs and 12,500 cs oils have not proven to be effective. In these induction experiments free silicone gel is injected into the peritoneal cavity. Much of the gel remains in a single mass that lies unattached in the peritoneal space; however, small fragments and liquids can break away and become incorporated into a silicone granuloma that forms chiefly on mesenteric surfaces. This mobile gel material appears as vacuoles in histological sections. The connective tissues that form around these gel fragments contain numerous inflammatory cells including lymphocytes and plasma cells. The tissue response to 1000 cs silicone oils is strikingly different. Though scattered lym phocytes and plasma cells can be seen, the vacuoles are much more tightly packed together and the striking intervacuolar inflam matory component seen with gels is much diminished or absent.
