DTIC ADA424262: Peptide-Mediated Transduction of Proteins and Nucleic Acids to Prevent and Treat Experimental Prostate Cancer PDF
Preview DTIC ADA424262: Peptide-Mediated Transduction of Proteins and Nucleic Acids to Prevent and Treat Experimental Prostate Cancer
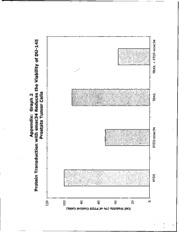
, 41" AD_____ Award Number: DAMD17-02-1-0129 TITLE: Peptide-Mediated Transduction of Proteins and Nucleic Acids to Prevent and Treat Experimental Prostate Cancer PRINCIPAL INVESTIGATOR: Janey D. Whalen, Ph.D. CONTRACTING ORGANIZATION: University of Pittsburgh Pittsburgh, PA 15260 REPORT DATE: January 2003 TYPE OF REPORT: Annual PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland 21702-5012 DISTRIBUTION STATEMENT: Approved for Public Release; Distribution Unlimited The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation. 20040706 048 Form Approved REPORT DOCUMENTATION PAGE OMB No. 074-0188 Public reporting burden for this collection of information is estimated to average I hour per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing this collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Washington Headquarters Services, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188), Washington, DC 20503 1. AGENCY USE ONLY 2. REPORT DATE 3. REPORT TYPE AND DATES COVERED (Leave blank)I January 2003 Annual (1 Jan 2002 - 31 Dec 2002) 4. TITLE AND SUBTITLE 5. FUNDING NUMBERS Peptide-Mediated Transduction of Proteins and Nucleic Acids DAMD17-02-1-0129 to Prevent and Treat Experimental Prostate Cancer 6. AUTHOR(S) Janey D. Whalen, Ph.D. 7. PERFORMING ORGANIZATION NAME(S) AND ADDRESS(ES) 8. PERFORMING ORGANIZATION REPORT NUMBER University of Pittsburgh Pittsburgh, PA 15260 E-Mail: jwhalet@imap .pitt. edu 9. SPONSORING / MONITORING 10. SPONSORING / MONITORING AGENCY NAME(S) AND ADDRESS(ES) AGENCY REPORT NUMBER U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland 21702-5012 11. SUPPLEMENTARY NOTES 12a. DISTRIBUTION / AVAILABILITY STATEMENT 12b. DISTRIBUTION CODE Approved for Public Release; Distribution Unlimited 13. ABSTRACT (Maximum 200 Words) Our goal in this project is to prevent the occurrence of bone metastasis in early experimental prostate cancer using protein transduction: the ability of small peptides to facilitate the entry of large biologically active fusion protein cargos into cells. The hypothesis to be tested is that protein transduction can deliver therapeutic proteins to skeletal tissues and bone marrow in such a manner that they may facilitate the apoptotic, or programmed cell death of cancerous cells and tissues of the bone. Prostate cancer.', lethal and incurable once it has metastasized to the bone. We and others have previously shown that protein transduction can allow many proteins into organs previously unaccessible to other delivery methods (drugs, viral vectors etc). We have constructed unique protien-transduction domains (PTD5 and Lys8) which we have demonstrated efficiently facilitate the entry and activity of the pro-apoptotic peptide Smac34 into prostate cancer cell lines. Protein transduction may prove to be a useful drug delivery method of biologically active proteins in cancer. J 14. SUBJECT TERMS 15. NUMBER OF PAGES 10 drug delivery; protein therapy; prostate cancer; bone 16. PRICE CODE 17. SECURITY CLASSIFICATION 18. SECURITY CLASSIFICATION 19. SECURITY CLASSIFICATION 20. LIMITATION OF ABSTRACT OF REPORT OF THIS PAGE OF ABSTRACT Unclassified Unclassified Unclassified Unlimited NSN 7540-01-280-5500 Standard Form 298 (Rev. 2-89) Prescribed by ANSI Std. Z39-18 298-102 Table of Contents C over ................................................................................................ I SF 298 ............................................................................................ 2 Table of Contents ........................................................................... 3 Introduction ................................................................................... 4 Body .............................................................................................. 4 Key Research Accomplishments .......................................................... 7 Reportable Outcomes ..................................................................... 7 Conclusions ....................................................................................... 7 References .................................................................................... 8 Appendices .................................................................................... 9 INTRODUCTION: Our goal in this project is to prevent the occurrence of bone metastasis in early experimental prostate cancer with systemic protein therapy via protein transduction. Protein transduction describes the ability of small regions of proteins, called protein transduction domains (PTDs), to facilitate the entry of large biologically active fusion proteins into the cell lines in vitro, as well as cells and tissues of experimental animals (mice) in vivo. The hypothesis to be tested is that protein transduction can deliver therapeutic proteins to the bone marrow, periosteum, adjacent skeletal muscle and to the bone (osteocytes). Delivery of proteins in such a manner may facilitate the apoptotic, or programmed, cell death of cancerous cells and tissues of the bone. The first specific aim (Task #1 & #2; Statement of Work) of this project is to demonstrate the ability of protein transduction domain (PTD)-mediated protein delivery to halt prostate cancer cell line proliferation in vitro, and to prevent and halt tumor development within and throughout the bone of immuno-incompetent mice in an established xenograft model of human prostate cancer in bone. The second specific aim (Task #3 & #4) is to develop an immuno-competent murine model of prostate cancer metastasis in order to ascertain the effects of PTD-based therapy on the development, of any, autoimmune responses. BODY: The first specific aim (Task #1 & #2; Statement of Work) of this project is to demonstrate the ability of protein transduction domain (PTD)-mediated protein delivery to prevent and halt tumor development within and throughout the bone of immuno-incompetent mice in an established xenograft model of human prostate cancer in bone. A. Task #1 was to transduce the PC3 prostate cancer tumor cell line with p53 protein, a tumor suppressor proteiri, via protein transduction in vitro. This has been accomplished: Rationale: The PC3 cell line was derived over 30 years ago from the bone metastasis of a 56 year old man with prostate cancer. The PC3 cell line does not contain the tumor suppressor p53 protein (i.e. p53 null). Our hypothesis for Task #1 is that introduction of the p53 protein into PC3 cells will provide the cells with the missing tumor suppressor protein, thus halting cell growth and proliferation of the tumor cell line. 4 The following construction of the protein transduction domain coupled to the p53 protein was carried out in the laboratory of our collaborator, Paul D. Robbins, Ph.D (University of Pittsburgh). i. An expression plasmid was constructed with the gene encoding the human p53 protein. ii. The expression plasmid was cloned and expressed in insect cells and the presence of p53 protein was confirmed in cell lysates by Western immunoblot analysis. The resulting band appeared to be identical to wild-type, purified human p53 (positive control). iii. The expressed p53 protein was chemically coupled to the PTD5 protein transduction domain (a novel PTD developed in our collaborators laboratory). The p53 protein was also separately coupled to the HIV Tat protein transduction domain (demonstrated by numerous investigators and reported in the literature to efficiently shuttle protein cargos into cells). An additional construct consisted of PTD5-coupled to a marker protein, beta-galactosidase. Evaluation of the ability of PTD5-p53 to transduce p53-null PC3 prostate cancer cell line and halt its -growth in vitro: PTD5-beta-galactosidase marker protein efficiently entered PC3 (prostate-cancer derived) cells with almost 100 % efficiency (at the higher doses) and entry into the cells was dose dependent. PTD5-beta galactosidase efficiency of transduction into PC3 cells was almost identical to that of the positive control HIV-TAT protein transduction domain. These results demonstrated that the PTD5 protein transduction domain was capable of transducing the PC3 prostate cancer cell line in vitro. These data are consistent with experiments using PTD5-beta-galactosidase in other (non-prostate cancer) cell lines as well as primary cell cultures. Additional constructs were also used, for example, PTD-4, 3, 2 and 1. These constructs were 8-12 amino acids long, and contained various numbers of lysines or arginines, amino acids hypothesized to be important in protein transduction efficiency. None of these alternative PTDs had the ability to transduce PC3 prostate cancer cell lines carrying the beta-galactosidase marker protein as well as PTD-5 or the positive control HIV- TaT protein transduction domains. The PTD5-p53 and the TAT-p53 constructs were incubated with PC3 prostate cancer cells at various concentrations and the effect on cell proliferation was assessed colorimetrically with MTT (3-(45- dimethyl-2-thiazolyl)-2,5, diphinyltetrazolium bromide, Sigma). Unfortunately, there was no effect on PC3 prostate cancer cell proliferation whatsoever, even at relatively high concentrations of PTD5 or HIV-TAT p53 protein transduction domains. These data suggested that the p53 protein biological activity may have been compromised during coupling to either of the two experimental transduction domains. Further attempts were made to resynthesize the p53 protein and couple it to PTD5 or HIV-TAT protein transduction domains. Additional attempts were made to couple active portions of the p53 protein (smaller bioactive peptides) to the PTDs. No construct, in our hands, was ever found to be capable of arresting the cell cycle or cause apoptosis of the PC3 cell line (or any other p53 null cell line). Apparently, we are not the only laboratory who has experienced difficulty in obtaining functional activity of protein transduction domains carrying cargos of p53 or p53 peptides. Personal communications with Dr. Steven Dowdy (Swartze et al. Science 1999), reported inconsistencies in p53 activity following fusion of the p53 protein to the HIV-Tat related protein transduction domain. To test the hypothesis that PC3 cell growth could nonetheless be halted via alternative PTD-mediated cargos, we tested the ability of a PTD5-smac construct created in our collaborator's laboratory (P.D. Robbins). SMAC is the abbreviation for 'second- mitochondria-derived activator of caspases. SMAC is considered to be a master regulator of apoptosis, 6r programmed cell death in mammals. Specifically, smac induces the activation of procaspase- 3 and promotes the enzymatic potential of mature caspase-3. Caspases are crucial regulators of apoptosis. PTD5-smac34 was constructed as outlined above, and consisted of the PTD5 protein construction domain and 34 amino acids from the amino-terminus of the smac protein. We found that the DU 145 cell line was the most susceptible to PTD5-smac34-induced cell death, as assayed by MTT. A dose dependant effect was demonstrated when PTD5-smac34 was added at various concentrations (25-200 uM) ;(graph 1; Appendix. 6 Furthermore, we also found that the PTD5-smac34 construct did not require TRAIL (Apo-2L/tumor necrosis factor-related apoptosis- inducing ligand) for its activity. Prostate cancer cell lines cultured with PTD5-smac34 viablity was only slightly reduced when TRAIL was added to the cultures. Under normal conditions, TRAIL is necessary for the release of smac from mitochondria via translocation of BAX from the cytosol to the mitochondria. In our experiments, PTD5-smac34 did not need TRAIL to induce prostate cancer cell apoptosis (graph 2; Appendix). KEY RESEARCH ACCOMPLISHMENTS: " Task #1 was to transduce the PC3 prostate cancer tumor cell line with p53 protein, a tumor suppressor protein, via protein transduction in vitro. This has been accomplished. " Due to unexpected difficulties in obtaining biological activity with PTD- fusion protein to the tumor suppressor protein p53, we successfully constructed and utilized an alternative, the PTD-smac fusion protein, to induce apoptosis (programmed cell death) in prostate cancer cell lines in vitro. REPORTABLE OUTCOMES: 1. Abstract of this work was presented at the 9th Annual Scientific Retreat held September 20-22nd in Washington, DC. The title of the Abstract was: Treatment of Experimmental Prostate Cancer via Protein Transduction of a PTD- 5/smac-trail fusion peptide. CONCLUSIONS: Protein Transduction domains have been described in nature (Elliot & Ohare, 1997; Joliot et al. 1991; reviewed in Swartze et al. 2000) and have been constructed from highly cationic amino acids (Mi et al. 2000). Essentially protein transduction domains are small peptide domains that freely cross cell membranes. Cargos of proteins linked to protein transduction domains can be transferred directly to cells. 7 We have found that the protein transduction domain constructed by our collaborator (Mi et al. 2000) is highly efficient at facilitating the delivery of marker proteins (e.g. beta-galactosidase) to prostate cancer cells in vitro. Unfortunately, it appears that all proteins are not as amenable to such treatment. We have concluded from our experiments reported here that the tumor suppressor protein, p53, is biologically inactive when transported to cells via protein transduction. It appears that this is due to improper folding of the protein following covalent linkage to the protein transduction domain. Our findings are not unique; other groups using similar, yet different transduction domains have had similar results (personal communications with various scientists). We are now preparing to employ the PTD5-smac34 in vivo as part of Task #2 (Specific Aim 1). We will treat established tumors in vivo with direct and systemic injections of PTD5-smac34 constructs at 50 uM. We also anticipate the synthesis of additional tumor suppressor/PTD5 combinations such as p16 and will employ these as they become available. REFERENCES: Elliott G, O'Hare P: Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 88:223-233, 1997 Hawiger J: Noninvasive intracellular delivery of functional peptides and proteins. Curt Opin Chem Biol 3:89-94, 1999 Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A: Antennapedia homeobox peptide regulates neural morphogenesis. Proc Natl Acad Sci U S A 88:1864- 1868, 1991 Mi Z, Mai J, Lu X, Robbins PD: Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitr6 and in viva. Mol Ther 2:339- 347, 2000 Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF: In viva protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569-1572, 1999 Schwarze SR, Hruska KA, Dowdy SF: Protein Transduction: unrestricted delivery into all cells? (Review) Trends Cell Biol 10:290-295, 2000 8 0 (U Sm (U o- 'I - (A CL C CL ( c Ln( a- W(U Vn 0 r- 0) o Ln0 (loiCuIm.L JOAu o/ 0)A411qelA118 In u LAE .C-. 0E CL IA+ 91 r.-m 4' U- / - XIA Ln 0 ) C00. 0C CB- o DV 4'I: OZO)S.d Oo0 4lql O
