DIRECT SEEDING OF BROSIMUM ALICASTRUM SW. (MORACEAE) AND ENTEROLOBIUM CYCLOCARPUM (JACQ.) GRISEB. (MIMOSACEAE) IN DIFFERENT HABITATS IN THE DRY TROPICS OF CENTRAL VERACRUZ PDF
Preview DIRECT SEEDING OF BROSIMUM ALICASTRUM SW. (MORACEAE) AND ENTEROLOBIUM CYCLOCARPUM (JACQ.) GRISEB. (MIMOSACEAE) IN DIFFERENT HABITATS IN THE DRY TROPICS OF CENTRAL VERACRUZ
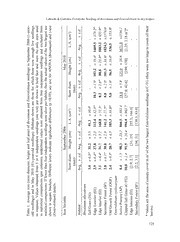
Acta Botánica Mexicana 100: 107-134 (2012) DIRECT SEEDING OF BROSIMUMALICASTRUM SW. (MORACEAE) AND ENTEROLOBIUM CYCLOCARPUM (JACQ.) GRISEB. (MIMOSACEAE) IN DIFFERENT HABITATS IN THE DRY TROPICS OF CENTRAL VERACRUZ & Javier Laborde1’ 2 Isabel Corrales-Ferrayola1 Tnstituto de Ecología, A.C.carretera antigua a Coatepec 351, El Haya, 91070 Xalapa, Veracruz, México. 2Autor para la correspondencia: [email protected] ABSTRACT Secondary forest in the seasonal tropics is usually dominated by a few pioneer tree species (usually wind-dispersed), while animal-dispersed species with large seeds may be absent. Several studies have shown that directly seeding these tree species in abandoned pastures can be successful; however, information is lacking about the optimal habitat We conditions for sowing. selected two large-seeded zoochorous canopy tree species that are cominon in the semi-deciduous tropical forest of central Veracruz, México: Brosimum alicastrum and Enterolobium cyclocarpum. Their seeds were sown in seven habitats: six fonning a gradient of increasing vegetation structure, from active pasture to 10-year-old We secondary forest, and an old-growth forest. assessed seed predation by granivores, protecting half of the seeds in wire cages. For a year we monitored seedling emergence, survival and growth, re-visiting the sites four-and-a-halfyears later. Seedling emergence was relatively high (75% in Brosimum 60% in Enterolobium) and fairly even among habitats. , Surprisingly, no seeds were removed by granivores. Enterolobium seedling survival and growth was higher in open habitats (around 60% survival up to a year) than in habitats shaded by woody plants (<10%). For Brosimum, the reverse was trae; its seedlings survived and grew betterunder a dense woody canopy (>80% survival) than in open sites (0%). Our results show that abandoned pastures and secondary forests can be successfully enriched by directly seeding poorly-dispersed forest canopy tree species, ifthe right habitat for sowing is chosen with care and based on the ecology ofseedling establishment ofthe desired species. Key words: forest recovery, large-seeded trees, secondary forest, seed predation, seedling establishment, semi-deciduous tropical forest. 107 Acta Botánica Mexicana 100 107-134 2012 : ( ) RESUMEN Los acahuales (i.e. selvas secundarias) de zonas tropicales secas o estacionales, suelen serpobres en especies arbóreas y dominados porunas cuantas especies de arbustos y árboles pioneros, usualmente dispersados por viento, siendo notable la ausencia de plantas arbóreas del dosel cuyas semillas relativamente grandes dependendevectores animales para su dispersión. Varios estudios han encontrado que la siembra directa de especies arbóreas zoócoras con semillas grandes, en pastizales abandonados puede ser una práctica exitosa para enriquecer y acelerar la sucesión secundaria. Sin embargo, todavía no se conocen cabalmente las condiciones de hábitat o etapa sucesional óptima para realizar la siembra directa de semillas de árboles de fases sucesionales tardías. En este estudio seleccionamos dos especies arbóreas con semillas zoócoras relativamente grandes, que suelen formarparte del dosel de las selvas sub-caducifolias del centro de Veracruz: Brosimum alicastrum y Enterolobium cyclocarpum. Las sembramos en siete hábitats; seis de ellos representando un gradiente de menor a mayor complejidad estructural o desarrollo sucesional, desde pastizal activo hasta acahual de 10 años y el hábitat restante fue selva mediana sub-caducifolia bien conservada. Evaluamos experimentalmente la importancia de la depredación de semillas, protegiendo la mitad de ellas sembradas dentro de jaulas diseñadas para excluir a vertebrados granívoros. Durante un año monitoreamos la emergencia, supervivencia y crecimiento de plántulas, marcando a las que sobrevivieron su primer año, para ser registradas cuatro años y medio después. Un porcentaje relativamente alto de plántulas emergió de las semillas sembradas (75% en Brosimum, 60% Enterolobium). No detectamos diferencias significativas en la emergencia de plántulas entre los siete hábitats, ni entre los dos tratamientos de exposición a granívoros (dentro vs. fuera de exclusorios). Ninguna de las semillas sembradas fue removida por granívoros. La supervivencia y crecimiento de Enterolobium durante el primer año fue mayor en hábitats abiertos sin cobertura de plantas leñosas (ca. 60%) que en los sombreados por arbustos y árboles (<10%). En contraste, las plántulas de Brosimum sobrevivieron y crecieron mucho mejor bajo la sombra de plantas leñosas (>80%) que en hábitats abiertos (0%). Nuestros resultados muestran que los pastizales abandonados y los acahuales pobres en especies arbóreas, pueden ser enriquecidos mediante la siembra directa de árboles de fases sucesionales tardías con baja capacidad de dispersión, siempre y cuando se elija cuidadosamente el hábitat (o etapa sucesional) óptimo para la siembra de semillas, con base en la ecología del establecimiento de plántulas de las especies involucradas. En el centro de Veracruz se puede acelerar la recuperación de la selva, sembrando semillas de Enterolobium desde el momento del abandono del pastizal, siempre y cuando se controle el crecimiento de los pastos durante A los primeros dos a tres años de crecimiento de las plántulas. su vez las semillas de 108 & Laborde Corrales-Ferrayola: Seeding ofBrosimum andEnterolobium in dry tropics Brosimum deberán sembrarse hasta que los arbustos o árboles pioneros hayan colonizado el sitio y sombreado a los pastos. Palabras clave: árboles de semillas grandes, depredación de semillas, establecimiento de plántulas, regeneración forestal, selva mediana sub-caducifolia, selva secundaria. INTRODUCTION Over the last six decades an unprecedented area ofold-growth and secondary forest has been cleared in the tropical Americas, most ofwhich has been converted & into pastures (Toledo, 1989; Chazdon, 2003; Griscom Ashton, 2011). Concomitant with this massive deforestation is the severe fragmentation of the remaining tropi- cal forest, which has left forest fragments of different sizes scattered in a landscape matrix of pastures and other deforested areas. The situation is so extreme that the very existence oftropical forests is injeopardy in several regions (Challenger, 1998; Terborgh, 1999; Laurance et al., 2006). Clearly, deforestation has to be stopped and the reserves set up to protect tropical forest need to work properly. However, both of these strategies need to be complemented by the restoration of tropical forest in degraded areas if we want to achieve the long term persistence of tropical forest ecosystems and preserve their impressive biodiversity (Terborgh, 1999; Chazdon, & 2003; Laurance et al., 2006; Griscom Ashton, 2011). Regrettably, the restoration of tropical forest in sites previously used as pas- tures is not an easy task. Pasture management practices, including grazing by cattle, quickly exhaust the re-sprouting potential ofthe roots and stumps ofwoody plants, also depleting the soil seed bank ofwoody plants (Reliman, 1974; Holl, 1999; Jan- zen, 2002). Therefore, the main and often the only route by which a tree or any other forest plant might establish in a pasture is the immigration (dispersal) ofa seed into the site from a nearby seed source. However, due to the large size ofpastures, suit- able seed sources (i.e. forested sites) are usually too far away from the pasture to be effective. Furthermore, a large proportion ofthe tropical woody flora strictly depend on frugivorous animáis for seed dispersal and most ofthese animáis are reluctant to leave a forest patch and move into pastures (Holl, 1999; Janzen, 2002; Guevara et al., 2005). Consequently, secondary forests that grow on abandoned pastures are usually poor in tree species, and dominated by a handful ofpioneer trees which produce an abundance of small seeds dispersed by the frugivorous birds and bats that are habi- & tat generalists (Janzen, 2002; Guevara et al., 2005; Muscarella Fleming, 2007). 109 Acta Botánica Mexicana 100 107-134 2012 : ( ) In tropical dry forest an important proportion of the tree flora is anemochorous (wind-dispersed), and their seeds can reach pastures much more easily than those of A zoochorous (animal dispersed) tree species. first wave ofanemochorous trees that colonizes a pasture may dominate the site and delay the recruitment of zoochorous & tree species for several decades (Janzen, 2002; Griscom Ashton, 2011). Once a tree seed arrives at a pasture it has to overeóme new and different obstacles. The seeds could be heavily preyed upon by the granivorous animáis that & are abundant in abandoned pastures (Doust et al., 2006; García-Orth Martínez- Ramos, 2008) or the emerged seedlings might be eaten by a variety of herbivores (Holl & Quiros-Nietzen, 1999; Griscom et al., 2005). Those seedlings that escape predators will have to compete with fast-growing grasses and other herbaceous plants, which might easily out-compete them (Hooper et al., 2002; Griscom et al., 2005; Ortega-Pieck et al., 2011). In addition to these two biotic barriers, tree seeds and their seedlings also have to cope with the harsh micro-environmental conditions & prevalent in pastures that might hinder recruitment (Aide Cavelier, 1994; Holl, 1999; Hooper et al., 2002; Janzen, 2002; Chazdon, 2003). Recent restoration efforts as well as studies on tropical secondary succession indicate that, without assistance, in many cases late successional species might not be able to establish; in particular, large-seeded zoochorous tree species may fail to reach the site or take a very long time to colonize it (Holl, 1999; Janzen, 2002; & & Florentine Westbrooke, 2004; Doust et al., 2006; Bonilla-Moheno Holl, 2010; Colé et al., 2011). The enrichment of abandoned pastures and secondary forest with preferred tree species can be done in two ways: by transplanting seedlings produced in nurseries and by directly sowing their seeds (direct seeding) into the plot. The first has been shown to be successful in several studies (see reviews by Floren- & & tine Westbrooke, 2004; Griscom Ashton, 2011), however it is very expensive and usually limited to the few tree species that, owing to their high commercial or agricultural valué, are available in nurseries (Colé et al., 2011). Direct seeding, on the other hand, is much cheaper and has been used more frequently in recent years (Campana-Camargo et al., 2002; Hooper et al., 2002; Doust et al., 2006; García- & & Orth Martínez-Ramos, 2008; Bonilla-Moheno Holl, 2010; Colé et al., 2011). Although several studies have shown that direct seeding is a promising practice for accelerating forest succession and enriching secondary forests, there have been some contradictory results. In most of the studies in which the sown seeds failed to become seedlings there was no explicit control of seed predation and so it is not possible to know whether establishment failure was due to seed predators or to un- suitable micro-environmental conditions. Additionally, most of the direct seeding 110 Laborde & Corrales-Ferrayola: Seeding ofBrosimum andEnterolobium in dry tropics studies have been done in recently abandoned pastures, with only a few in secondary forests that vary in age, vegetation structure and microclimate (Campana-Camargo & et al., 2002; Bonilla-Moheno Holl, 2010; Colé et al., 2011). Also most of these studies have been done in the humid tropics. Thus, more information is needed on whether direct seeding can be equally successful when carried out in different types of habitat, particularly in more seasonal or drier tropical regions, and whether the tree species ofoíd growth forest have different optimal habitats where their seedling survival and growth are maximized. In addition, we need to discern to the best of our ability, the reasons that sown seeds fail to produce successful seedlings in dif- ferent habitats and circumstances. For this study, we selected Brosimum alicastrum and Enterolobium cyclocarpum two large-seeded zoochorous tree species that are common in the , canopy of the original semi-deciduous tropical forest of central Veracruz, México. Seeds ofboth species were sown in seven habitats that have contrasting vegetation cover and composition; six of them ranging from active pasture to a 10-year-old secondary forest. As a reference forest we included a remnant of old-growth forest We that has been protected since 1977. explicitly assessed the importance of seed predation by granivores, protecting half of the sown seeds inside wire cages. Over the course of a year we monitored seedling emergence, survival and growth, care- We fully recording the causes ofseedling mortality in each ofthe seven habitats. re- visited the sites to make final measurements ofgrowth and survival four-and-a-half years later. Our aim was to compare seedling performance among the seven habitats and determine if there is an optimal habitat for direct seeding of large-seeded can- opy tree species, and to assess whether the two tree species have different optimal habitats for seedling recruitment. If seed removal by granivores is a major factor preventing the establishment of sown seeds, then we expect seedling emergence to be higher inside the wire cages than outside them. The intensity of seed removal may vary widely among the habitats studied, in accordance with the habitat preferences and densities of seed predators: in grass dominated habitats we expect a high rate of seed removal by rodents, however in the forest of the study site it has been reported that the red land crab Gecarcinus lateralis) removes tree seeds and recently emerged seedlings ( from the forest floor (including Brosimum). Our results will reveal in which habitats seed predation is a strong impediment to direct seeding. Since Brosimum seeds are highly nutritious and unprotected, while those of Enterolobium are protected by a woody testa and not as palatable to granivores, we also expect a higher rate of seed removal for Brosimum. Differences in seedling survival and growth among habitats 111 Acta Botánica Mexicana 100 107-134 2012 : ( ) will reveal ifthere is an optimal habitat for sowing. Since differences in the floristic composition and vegetation structure ofthe seven studied habitats roughly resemble differences in forest recovery (i.e. secondary successional stage), the results of this study will show ifthere is an optimal moment in succession after pasture abandon- ment, when the selected species should be sown. Additionally, we did a field ma- nipulation experiment during the first year ofthe study, removing the grass foliage from a grassland site without cattle, in order to minimize light competition between the grasses and emerged woody seedlings. This will tell us if this time consuming and demanding activity positively affects seedling growth or survival in abandoned pastures. METHODS Study site This research was carried out in the “Centro de Investigaciones Costeras La Mancha” (CICOLMA) biological station located on the coast ofthe Gulf ofMéxico in central Veracruz, México (19°35'50" N; 96°22'45" W) and managed by the Insti- Aw tuto de Ecología, A.C. (INECOL). Climate is tropical wet and dry or sub-humid 2 with summer rains (Koppen modified by García, 1981; cited in Moreno-Casasola, 2006). Mean annual precipitation is 1286 mm/year (range: 899 to 1829 mm/year), the driest months are November to May (<60 mm/month) and the wettest are June to September (>150 mm/month). Mean annual temperature is 25.6 °C; mean valúes of the coldest and hottest month are 21.1 °C (January) and 27.3 °C (June), respec- tively (Moreno-Casasola, 2006; unpublished data from 1981 to 2006 collected at the CICOLMA meteorological station). There is an extensive system ofPleistocene-age CICOLMA sand dunes in the región and at the station a fossil dune dating from the Late-Glacial age has been overrun by a large N-S arm ofa parabolic dune that is no more than a few hundred years oíd. In the fossil dune, sand-sized particles comprise 70 to 80% of the soil with noticeable clay formation, while in the recent dune soils are mostly puré sand (>95%). Both dune soils have very high water infiltration rates in comparison with most other soil types and both are also comparatively poor in & nutrients (Kellman Roulet, 1990; Moreno-Casasola, 2006). The biological station and its protected area were established in 1977 when its 71.4 ha were mainly undisturbed native vegetation. In 1995 an adjacent, irregu- lar 11.9 ha polygon (covered mainly by pastures) was added to the reserve. Cur- CICOLMA rently, covers 83.3 ha, and the main vegetation types are semi-deciduous 112 & Laborde Corrales-Ferrayola: Seeding ofBrosimum andEnterolobium in dry tropics tropical forest (43.7 ha) and Coastal dune scrub (24.5 ha), with the rest comprised of several small patches of different vegetation types, including wetlands and low stature tropical dry forest or scrub (Moreno-Casasola, 2006). In the area added to the reserve in 1995, there is a plot of approximately 6 ha (±200 x 300 m) that, prior its annexation, was used for over 20 years as a pasture for cattle and was planted with the African grass Panicum máximum. In 1995, the cattle were removed from this plot in order to start a long term study ofvegetation change. Ten years later, in m 2005 a secondary forest, 8 to 10 tall, had covered most ofthe plot, except for a 90 m x 60 patch in one ofthe corners that still was covered by grasses. Five ofthe seven habitats in which seeds were sown in this study, were located inside this experimen- tal plot (details below). The grassland patch within the 6 ha abandoned plot, allowed us to trace transects that represented a vegetation gradient of increasing structural complexity, from tree-less habitat covered by grasses, to habitats with incipient and recent tree colonization at the edge ofthe grassland-secondary forest, up to densely shaded habitat in the interior ofthe 10 year oíd secondary forest; which roughly re- sembles a successional gradient (Fig. 1). Species selected and experimental design The two species selected -Brosimum alicastrum and Enterolobium cyclocarpum- are among the most common and largest trees ofthe semi-deciduous tropical forest of the región. Brosimum (hereafter only the genus will be used) is a common canopy tree in both the humid and dry tropical forests ofMéxico and Cen- mm tral America. Its seeds (13 to 20 in diameter) are dispersed by vertébrate frugi- & vores, mainly bats and othermammals (Peters, 1991; Pennington Sarukhán, 1998). Enterolobium is a deciduous tree common in lowland tropical areas from México to the northern part of South America, and is also relatively common in moist and dry tropical forest. Even though it can form part of the canopy of oíd growth forest it is also common in open conditions, including savannas and pastures. Its presence in forested patches is usually taken as a sign ofpast disturbance and it is commonly & & regarded as a mid to late secondary species (Blain Kellman, 1991; Pennington mm Sarukhán, 1998; Williams-Linera et al., 2011). Enterolobium seeds (15 to 20 in mm length; 10 to 12 wide) are mainly dispersed by ruminants, including domestic livestock and occasionally by hoarder rodents (Janzen et al., 1985); hydrochory may also be an important dispersal mechanism where flooding occurs Enterolobium ( pods float; Hunter, 1989). Seeds were collected between mid-July and early-August in 2005. Most Brosimum seeds were collected from beneath the canopy of five large trees, and ca. 113 ) Acta Botánica Mexicana 100 107-134 2012 : ( ) b) AP TG EE El SF ;: P P P P :c P :: O ' C-TG i 1 Fig. 1. Sowing experimental design: a) Schematic vertical profile of the seven habitats where seeds were sown (x3 replicates or transects); b) Spatial arrangement ofsowing blocks in the habitats (OGF, not shown); c) Sowing block layout and treatments: Ba (Brosimum alicastrum Ec {Enterolobium cyclocarpum), cage or granivore exclosure (=p sowing ), ), point (•), pennanent stake (). 20% of them were collected under a compact group of mango (Mangifera indica trees which were being used as feeding roosts by bats. Seeds were carefully picked, choosing only those from which the fruit pulp had been completely removed by fru- givores and had recently fallen to the ground. Enterolobium pods were collected be- low the canopy ofthree large trees. At the time the seeds were collected, 25 to 30% of the ripe pods of the 2005 crop were still on the tree. Only large, recently fallen pods were collected. Seeds were extracted from the pods and cleaned under water to remove the thin pulp and only large, well formed seeds were selected. Seeds were sown in seven distinct habitats which represent a gradient ofveg- etaron cover and successional development from active pasture to 10-year-old sec- ondary forest, and a protected oíd growth forest (Fig. 1). The 6 ha experimental plot described above was central to the layout ofthe experimental sowing blocks ofthis study. The first habitat was an active pasture (i.e. a pasture with grazing cattle; here- 114 Laborde & Corrales-Ferrayola: Seeding ofBrosimum andEnterolobium in dry tropics after, AP) adjacent to the experimental plot and outside the limits of CICOLMA. The cattle were being raised using the management practices common in the región, with a stocking density of one adult cow per hectare (for more details see Moreno- Casasola, 2006). Pastures in the area have low densities of isolated shade trees and are dominated by an African grass Panicum máximum which when grazed properly , grows dense foliage 5 cm in height. When overgrazed, the cover ofP. máximum is reduced while that of ruderal herbs and native grasses Paspalum and Axonopus ( ) increase. The other five habitats were located inside the 6 ha experimental plot: two within the patch still covered by grasses, another two at the border between the secondary forest and this grassland patch, and another inside the secondary forest (Figs. la, Ib). The grassland patch was dominated almost exclusively by P. m máximum which in the absence ofcattle grows densely and surpasses 1 in height, , m We reaching up to 2 during the rainy season. called this the tall grass habitat (TG), and sowed seeds directly beneath the grass without disturbing Two meters away it. from TG, we established a paired sowing site where we regularly clipped the grass- land turf to keep it at a height less than 5 cm during the first year ofthe study (i.e. until September 2006) and called this the clipped tall grass (C-TG) habitat, where competition with the grass foliage was minimized. The next habitat was at the outer edge of the secondary forest, beneath the m canopy of the outer line of colonizing trees, less than 5 away from the open grassland, and we called this the edge exterior (EE) habitat. Seeds were sown in m EE beneath the single, sparse layer ofwoody cover (1-3 tall), under which sparse and relatively short (<20 cm tall) tufts of grass and heliophytic herbs (50 to 60% of ground cover) were growing, the rest was bare soil. The next habitat was the edge m interior (El), situated 10 to 15 away from the grassland, on the inside ofthe outer line oftrees that formed the forest edge. The sowing sites ofthis habitat were shaded m by several trees up to 5 tall, and on the ground heliophytic herbs and grasses were rare (<10% ground cover). The next habitat was the interior ofthe 10-year-old m secondary forest (SF) for which we placed the sowing sites more than 50 away m from the forest edge. The tree canopy at SF sites was 10 high, with a dense layer m of tree foliage 5 to 10 above the ground. The ground in SF sites was completely covered with leaflitter and devoid ofheliophytic herbs and grasses. Vegetation sam- pling in this secondary forest in 2006 (eleven years after abandonment) recorded 55 species ofwoody plants with a DBH >2.5 cm and a stem density of 12.6 to 18.8 m stems per 100 2 (unpublished data). Three anemochorous woody species -Diphysa robinioides Mimosa tricephala and Cedrela odorata- were dominant, accounting , for 36.6% of the abundance. A mere 10% of woody stems with DBH >2.5 cm be- 115 Acta Botánica Mexicana 100 107-134 2012 : ( ) mm longed to zoochorous tree species, all ofwhich have relatively small seeds (<5 in diameter), henee the need to enrich this stand with large-seeded zoochorous tree species. The seventh habitat was the interior of an und isturbed oíd growth forest CICOLMA m (OGF) that has been protected since 1977 in (-550 away from the OGF m other six habitats). Sowing blocks in were placed more than 70 away from m any canopy light gap or forest edge, and had a 20 tall primary forest canopy over m them that reached máximum foliage density between 12 and 18 above the ground. m Each of our experimental sowing blocks measured 2.5 x and consisted 1 m m m oftwo 1 2 square quadrats separated by 0.5 (Fig. le). Within each 1 2 quadrat, Brosimum and Enterolobium were sown in opposite corners placing six seeds of each in two rows ofthree sowing points, 10 cm apart from each other. At each sow- ing point one seed was carefully buried 1 cm below the ground. To protect seeds from vertébrate seed eaters, in one quadrat the seeds were protected by a rectangular wire cage measuring 30 x 20 cm and 20 cm in height with a 0.5 cm pore size (gra- nivore exclosure), and in the accompanying quadrat seeds were also buried but left unprotected. Four permanent stakes (Fig. le) marked the sowing block’s position and allowed for the exact re-location ofeach sowing point (i.e. all seeds sown). In the dif- ferent habitats, the sowing blocks were loosely arranged along three transeets, each extending from the grassland patch into the secondary forest interior (Fig. Ib), i.e. there were three replicates (sowing blocks) for each habitat. In total, 252 seeds were sown per species. The distance between replicates in the same habitat varied: 40 to m m 45 for the tall grass habitats (TG and C-TG); 50 to 55 for the forest edge habitats m (EE and El) and 90 to 100 for the OGF, SF and AP habitats. All seeds were sown from 19 to 22 ofAugust 2005. After sowing, each block was visited weekly during the lst month, then every 15 days from the 2nd to 4th month and then once per month until May 2006, the last survey ofthe first year was done in mid-September 2006. In May 2010, almost five years after sowing, the experimental blocks were re-visited for final inspection. It is important to mention that the active pasture (AP) was abandoned in 2008 and its owner stopped raising cattle in there, so in 2010 when we re-visited this site, it had been abandoned for 2 years. On every field visit, for each sowing point we recorded whether a seedling had emerged or not. Measurements for each emerged seedling were stem diameter (mm) at 3 cm above ground; height (cm) to the final meristem; and seedling leafarea (L.A. in cm2 estimated by counting the number of leaves in each ofthree size cat- ), egories (see below). The date ofthe field visit when a seedling was found dead was assumed to be the date of death for the survival analysis. When possible, the most likely cause ofdeath was recorded; i.e. herbivory by vertébrate or invertebrate; dehy- 116
