B.Pharmacy Final Year - Dr. Babasaheb Ambedkar Marathwada PDF
Preview B.Pharmacy Final Year - Dr. Babasaheb Ambedkar Marathwada
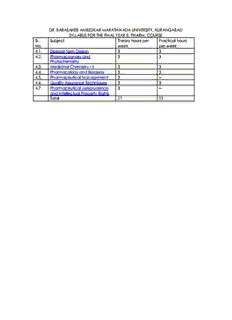
DR. BABASAHEB AMBEDKAR MARATHWADA UNIVERSITY, AURANGABAD SYLLABUS FOR THE FINAL YEAR B. PHARM. COURSE Sr. Subject Theory hours per Practical hours No. week per week 4.1. Dosage form Design 3 3 4.2. Pharmacognosy and 3 3 Phytochemistry 4.3. Medicinal Chemistry - II 3 3 4.4. Pharmacology and Bioassay 3 3 4.5. Pharmaceutical Management 3 -- 4.6. Quality Assurance Techniques 3 3 4.7. Pharmaceutical Jurisprudence 3 -- and Intellectual Property Rights Total 21 15 4.1 : Dosage Form Design – Theory (Final B. Pharm.) - 3 HRS. / WEEK Sr. Topics and contents Hours No. SECTION - A Preformulation: - 08 Concept of preformulation, 1. Organization of preformulation activity, Essential information of new drug for preformulation, Principle areas of preformulation: - Analytical preformulation, Bulk Characterization, Solubility, Stability Analysis. 2. Solubility and Dissolution: - 10 Methods of expressing solubility, Prediction of solubility Physicochemical prediction of solubility Solubility Parameters Factors affecting on solubility Dissolution mechanisms Factors affecting dissolution Intrinsic dissolution Measurement of dissolution rates 3 Stability: - 10 Definition: - Expiry date, Shelf life, Order of reaction. Environmental factors influencing stability: - pH, Solvent, Solubility, Additives, Light, Temperature, Acid Base Catalysis, Ionic Strength Modes of drug instability and methods to prevent them: - Chemical and Physical Technique of stability prediction, Tentative expiry dating Introduction to cGMP guidelines in stability testing and expiry dating(No details) Effect of packaging material on stability of drug. 4 Package Development: - 10 Structure and composition Glass- types, manufacture of glass, glass container, evaluation Plastic- definition, material properties (mechanical, electrical and optical), physicochemical properties (mass transfer, chemical attack), types-thermoplastic, thermoset with example and applications. Drug plastic interactions, evaluation of plastics Collapsible tubes- metal, plastic, lamination Closure: closure types (rubber, plastic), closure liners, tamper resistant packaging, film wrappers, blister packing material, strip package, bubble pack, shrink banding, pouches, bottle seal, taper seals, breakable caps, tape seals, seal cartons. SECTION B 5 Oral Sustained Release and Introduction to NDDS: - 15 Introduction, Terminology Biopharmaceutical aspects: - Steady state concepts, Calculation of loading and maintenance dose. Design of oral SRDF systems: - Biological factors, Physicochemical factors Diffusional systems: - Reservoir system, Lag time, Burst effect Matrix system, Effect of porosity and tortuosity Dissolution controlled system, Cube route dissolution equation, Diffusion layer controlled dissolution. Bioerodible and Combination of diffusion and dissolution systems Evaluation: - Official and unofficial methods of evaluation of SRDF/CRDF system Polymers used in SRDF system Introduction to NDDS( Novel Drug Delivery System): - Osmotically controlled system, osmotic pumps Ion exchange system Transdermal system Occular system Intravaginal and intrauterine system Injection and implant Biomucoadhesive system Iontophhoretic system Sonophoretic system Targeted drug delivery system: - Liposome, Nanoparticles, Prodrugs, Resealed erythrocytes, Antibody targeted systems 6 Scale Up Techniques: - 06 Introduction Steps involved in scale up General consideration Scale up examples of: Solid dosage form Liquid dosage forms- Solution, Suspension, Emulsion. Semi solid dosage forms- Suppository 7 Optimization and Experimental Design: - 07 Introduction Concepts of optimized drug product Various optimization parameters like problem types and variables Optimization techniques: - Classical optimization, Statistical design Optimization methods : Evolutionary, Simplex, Langarian, Search (Introduction only). Experimental design (Introduction to) Designing strategy and purpose of experimental design along with pharmaceutical examples. Stages in experimental design Starting of experimental design- Definition, Important concepts of experimental design, Software for designing. 8 Design of Dosage Form:- 06 Principles of dosage form design (DFD) Biopharmaceutical aspects of DFD Drug related factors in DFD Therapeutic considerations in DFD. References: • Leon Lachmann, H. Liebermann, “Principles and Practice of Industrial Pharmacy • Banker’s Modern Pharmaceutics • Pharmaceutical Experimental Design, , M and D series by Lewis Vol-92 • Kim, Advanced Pharmaceutics • Aulton, Pharmaceutics. • Rawland, Pharmaceutics. • “Remington’s Science and Practice of Pharmacy”, 20th edition, Vol-I and II • ICH guidelines for stability study • Indian Pharmacopoeia – Current Editions • British Pharmacopoeia – Current Editions • United State Pharmacopoeia – Current Editions • S.P.Vyas, R.K.Khar, Targeted and controlled drug delivery system, CBS Publication • Chein, Controlled Drug Delivery Systems, Marcel Dekkers Publication. 4.1 : Dosage Form Design – Practical (Final B. Pharm.) - 3 HRS. / Batch / Week 1 Compare oxidative degradation of ascorbic acid at pH 2.0 and 8.0. 2 Compare the degradation rate constant of ascorbic acid in presence of cuppric ions. 3 Construction and analysis of Plaquette Burman design to a hypothetical system. 4 Demonstration of fluidised bed processing technique. 5 Demonstration of mixing performance evaluation of mixer (Sigma/ Planetary/ ribbon/ RMG) 6 Demonstration of Preparation of microcapsules of given sample of drug by extrusion & sperodization technique. 7 Determination of compressibility of given material 8 Determination of effect of solvent on aspirin solution stability 9 Determination of F value of given dosage form 10 Determination of micromeritic properties of given material (MCC/ Starch etc.) 11 Determination of Phase solubility analysis of drug β-CD complex 12 Determination of solubility of given drug at different pH 13 Determine the energy of activation of hydrolysis of aspirin/ ascorbic acid solution 14 Determine the optimum dielectric constant for maximum solubility of drug. 15 Evaluate antimicrobial adsorption property of given sample of rubber closure. 16 Fitting of data to linear, log and polynomial system to determine best fit. 17 Perform pharmacopeia test for given sample of glass vial / ampoule. 18 Perform physicochemical test as per pharmacopoeia on given sample of plastic. 19 Preparation of ion exchange complex of given drug sample and evaluation of its release 20 Prepare & evaluate beads of given sample of drug using melt dispersion 21 Prepare & evaluate beads of given sample of drug using polymer coacervation 22 Study of effect of homogenizing time on emulsion globule distribution of liquid paraffin emulsion References: • Leon Lachmann, H. Liebermann, “Principles and Practice of Industrial Pharmacy • Banker’s Modern Pharmaceutics • Pharmaceutical Experimental Design, , M and D series by Lewis Vol-92 • Kim, Advanced Pharmaceutics • Aulton, Pharmaceutics. • Rawland, Pharmaceutics. • “Remington’s Science and Practice of Pharmacy”, 20th edition, Vol-I and II • ICH guidelines for stability study • Indian Pharmacopoeia – Current Editions • British Pharmacopoeia – Current Editions • United State Pharmacopoeia – Current Editions • S.P.Vyas, R.K.Khar, Targeted and controlled drug delivery system, CBS Publication • Chein, Controlled Drug Delivery Systems, Marcel Dekkers Publication. 4.2 : Pharmacognosy and Phytochemistry – Theory (Final B. Pharm.) - 3 HRS. / Week Section A 1. Phytochemical investigation of crude drugs and it’s importance (1) Detail study of each category of drugs under phytochemical scheme including biosynthesis 2. VOLATILE OIL AND TERPENOIDS: (12) Definition composition Chemistry and extraction of volatile oils, biosynthesis, terpeneless volatile oils Study of drugs containing volatile oils a) Monoterpenoids: Rosemary, Anise, Cummin, Celery, Lavender, Gaultheria, Palmarosa, Citronella, Thyme, Camphor, Chenopodium, Eucalyptous, Lemongrass, Turpentine, Pepprmint oil, Caraway, Cardamom, Coriander, Ajowan, Dill, Fennel, Lemon peel, Orange peel, Nutmg, Cassia, Cinnamon, jatamansi, garlic, Sweet basil, Sacred basil, Musk, Civet b) Sesquiteerpenoids: Artemisia, Davana, Arnica, Sandalwood, Clove, Hops, Saussurea, Cubeb, Valerian, Ferverfew c) Diterpenoids: Taxus, Coleus d) Triterpenoids: Ambergris e) Tetraterpenoids and polyterpenoids: Annatto, Saffron Structure determination and elucidation of, Citral, Geraniol, Menthol, Santonin, Beta carotene 3. ALKALOIDS: (12) Definition, distribution, classification, methods of isolation, properties, chemistry and biosynthesis of following group of alkaloids Study of alkaloid containing drugs, 1) Tropane: Belladonna, Datura, Hyoscamus, Stramonium, Coca, Duboisa 2) Indole: Ergot, Nuxvomica, Physostigma, Rauwolfia, Vinca, 3) Isoquinoline: Opium, Ipecac, berberis, Hydrastis, Curare, 4) Quinoline; Cichona, and Camptotheca 5) Pyridine: Lobelia, Areca 6) Quinazoline: Vasaka 7) Purine; Coffee, Tea, and Coca 8) Imidazole; Pilocarpus 9) Steroidal: Veratrum, Kurchi, Ashwagandha, 10) Protoalkaloids: Ephedra, Colchicum, Aconite, Gloriosa Structure determination and elucidation of, Reserpine, Morphine, Atropine, Caffeine, Ephedrine 4. MARINE DRUGS: (04) Indroduction, importance, classification of drug molecules from marine organisms a) Cytotoxic and antineoplastic agents b) Cardiovascular drugs c) Marine toxins d) Antimicribial drugs e) Antibiotic substances f) Antiinflammatory and antispasmodics g) Miscelleneous pharmacologically active substances Section B 1. Extraction isolation and analysis of phytopharmaceuticals (06) • A detailed study of various methods of extraction and isolation of phytopharmaceuticals namely infusion, decoction, digestion, maceration, percolation, successive solvent extraction, supercritical fluid extraction, steam distillation, had space techniques, sepbox, selection of suitable extraction process • Application of chromatography and spectroscopy to plant drug analysis 2. Industrial importance and status of herbal drugs (02) Role of medicinal and aromatic plants in national economy Importance and status of herbal medicines, aromatics and cosmetics 3. Worldwide trade in medicinal and aromatic plants and their derived products. A brief account of plant based industries and institutions involved in work of medicinal and aromatic plants and their products in India (02) 4. Details of methods of isolation including industrial identification, chemistry and estimation of, a) Quinine b) Ephedrine c) Cardiac glycosides d) Ca sennosides e) Diosgenin f) Glycyrrhizin g) Citrus bioflavonoids h) Rutin i) Andrographolides j) Phyllanthin k) Guggulosterol l) Gymnrmic acid m) Asiaticoside/ madecassoside n) Withanolides (10) 5. Standardization of herbal drugs: (05) i) Importance of standardization of raw materials, extracts and formulation ii) problems involved in standardization of herbs iii) Standardization of single drug and compound formulation iv) Estimation of parameter limits used for standardization v) Herbal extracts vi) WHO guidelines or quality standardization of herbal formulation 6. HERBS AND HEALTH FOODS: (10) • Introduction • Nutraceuticals, Antioxidants, Prebiotics and probiotics, Poluunsaturated fatty acids, (PUFA), Dietry fibres • Definition Introduction status, safety quality control and efficacy and brief study of herbs such as, Alfalfa, Angelica, Arnica, Apricot, Borage, Bran, Chamomile, Chicory, Colcellana, Cucumber, Damiana, Devil’s claw, Fenugreek, Garlic, Onion, Gentian, Ginseng, Ginkgo biloba, Hydrocotyl, Golden sal, Hibiscus, Hops, Honey, Kelp, Marigold, Mormontea, Parsley, Passiflora, pennyroyal, Soyabeen, Ganoderma fruits, evening primrose, corn oil, Tejpat oil, Blessed thistle, Balm lemon foliage and flower a. Herbal formulations: (05) A comparative study of traditional and modern dosage forms. Classification general considerations and different stages of herbal formulations and dosage forms b. Herbal cosmetics: (05) Definition, classification and role and importance of herbs in cosmetics Study of following herbal cosmetics i. Shampoo- soapnut ii. Conditioner- Amla, Henna, Hibiscus and Tea iii. Hair darkners; Amla and Henna iv. Skin care: Aloe vera, Turmeric, Sandalwood, Glycyrrhizin v. Herbal soap vi. Herbal mouthwash vii. Herbal hair tonic viii. Liquid cream ix. Lotion c. Patenting of natural products (02) Different aspects of obtaining patents in natural products (rules and regulations therein)
Description: